ISSN 2581-5369
HeinOnline, MANUPATRA, Google Scholar Indexed

Trial by Media: An Overview
- Nikitha Suresh and Lucy Sara George
- Show Author Details
Nikitha Suresh
Student at Kerala Law Academy Law College, India
Lucy Sara George
- img Download Full Paper
- img Export Citation
Export citation
Trial by media is a phrase popular in the late 20th century and early 21st century to describe the impact of television and newspaper coverage on a person's reputation by creating a widespread perception of guilt or innocence before, or after, a verdict in a court of law. In recent times there have been numerous instances in which media has conducted the trial of an accused and has passed the verdict even before the court passes its judgment. The Supreme Court reiterated that the media and the judiciary are institutions inhabiting separate spheres and their functions do not overlap. One cannot and must not use the other for discharge of its functions. It was observed that media should only engage in acts of journalism and not act as a special agency for the court. The impermissibility of freedom of speech and expression amounting to interference with the administration of justice due to the prejudicial nature of certain media coverage is highlighted through this paper.
- media trial
- fourth pillar
- click-bait journalism
- miscarriage of justice
Research Paper
Information
International Journal of Law Management and Humanities, Volume 4, Issue 2, Page 267 - 272
Creative Commons

This is an Open Access article, distributed under the terms of the Creative Commons Attribution -NonCommercial 4.0 International (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/), which permits remixing, adapting, and building upon the work for non-commercial use, provided the original work is properly cited.
Copyright © IJLMH 2021
I. Introduction
Media is considered to be the fourth pillar of democracy, after Legislature, Executive and Judiciary. Media as fourth pillar was coined by Thomas Caryle.
A responsible press is the handmaiden of effective judicial administration [1] . The press does not simply publish information about cases and trials but subjects the entire hierarchy of the administration of justice (police, prosecutors, lawyers, judges, courts), as well as the judicial processes, to public scrutiny. Free and robust reporting, criticism and debate contribute to public understanding of the rule of law, and to a better comprehension of the entire justice system. It also helps improve the quality of that system by subjecting it to the cleansing effect of exposure and public accountability. “Sunlight” as Justice Brandeis once said “is the best of disinfectants, electric light the most efficient policeman.” [2]
II. Laws governing media in india
There was regulation for Press until the British East India Company began ruling a portion of India in 1757 after the Battle of Plassey. The enactment of the Press and Registration of Books Act, 1867 was a very significant event in the field of laws governing Media. The aforementioned Act is still in force and the same was enacted to regulate the printing press along with periodicals which contained news, further the objective of the act was to preserve copies of books and for the registration of Books.
In 1869-70, when Media played a huge rule during the Wahabi Conspiracy, Sedition was incorporated as an offence as Section 124 A in the Indian Penal Code, 1870 wherein exciting or even attempting to excite any feeling of disaffection/feeling of enmity to the Government was labelled as an offence which as of today, is punishable with imprisonment of life to which fine maybe added. In pursuant to the above, the Dramatics Performances Act, 1876 was brought into force so as keep a check on public dramatic performances which had the possibility of provoking people against the Government. When the then Government sensed the press becoming bold by use of their Indian Language, so as to ascertain and achieve “better control” of the language press, the Vernacular Press Act, 1878 was enacted and brought into force.
In 1851 the telegraph was introduced, pursuant to which the Indian Telegraph Act was enforced in 1885. Consequently, the then Government in 1908 passed the Newspaper (Incitement to Offences) Act which empowered the local authorities to take an action against editor of any newspaper wherein it was suspected/observed that the articles contained in the newspaper, had the tendency to incite rebellion. Subsequently, the Press Act, 1910 was enforced wherein the Government was authorised/empowered to claim an amount under the garb of security from any Newspaper. In furtherance, to the aforementioned act, the Government enacted/passed the Copyright Act ,1957 and the Cinematograph Act in 1952.
Lately, the Right to Information Act was introduced in 2005 and the implementation of the same has stretched out the freedom of press which made India a liberal country, when it comes to Freedom of Press. There are numerous laws that control and regulate the performance of Press in India. The Constitution of India,1950 has not laid down any specific provision for the Freedom of Press separately but the same can be derived from Article 19(1)(a) of the Constitution of India,1950 which guarantees Freedom of Speech and Expression to the citizens of India. Article 19(1) (a) of the Constitution of India 1950
‘Trial by media’ is a phrase popular in the late 20th century and early 21st century to describe the impact of television and newspaper coverage on a person’s reputation by creating a widespread perception of guilt or innocence before, or after, a verdict in a court of law. In recent times there have been numerous instances in which media has conducted the trial of an accused and has passed the verdict even before the court passes its judgment. Some famous criminal cases that would have gone unpunished but for the intervention of media, are Priyadarshini Mattoo case , Jessica Lal case , Nitish Katara murder case and Bijal Joshi rape case [3] .
III. Judicial decisions
The Hon’ble Supreme Court in the many cases has ruled that freedom of press is a fundamental right covered by the right to freedom of speech and expression. In the case of Brij Bhushan v. State of Delhi [4] , held that in India under Art.19(1)(a) freedom of speech and expression authoritatively includes the freedom of press print and electronic media and affecting the right of freedom of speech and expression.
And in the case of Romesh Thapar v. State of Madras [5] , Supreme Court held that freedom of speech or freedom of press lays the foundation of all the democratic organization without political discussion, no public education is possible which is necessary for proper functioning of popular government. In the case of India Express Newspaper Ltd. v. Union of India [6] , Justice Venklatrana of Supreme Court of India sated that the freedom of press is an essential for the proper functioning of the democracy.
In LIC v. Manubbai Shah [7] , the Supreme Court reiterated that the freedom of speech and expression must be broadly construed to include the freedom to circulate one’s views by word of mouth, or in writing, or through audio visual media. This includes the right to propagate one’s views through the print or other media. The Apex Court observed: “Freedom to air one’s view is the lifeline of any democratic institution and any attempt to stifle, or suffocate, or gag this right would sound a death knell to democracy and would hold usher in autocracy or dictatorship.”
In the case of Printers (Mysore) Ltd. v. Assistant Commercial Trade Officer [8] , the Supreme Court of India held that though freedom of press is not under Fundamental Right, but it is an implicit in the freedom of speech and expression. In R.Rajagopal v. State of Tamil Nadu [9] , the Supreme Court held that neither the Government nor the officials had any authority to impose a prior restraint upon publication of a material on the ground that such material was likely to be defamatory of them. In Re: Vijay Kumar [10] , the Supreme Court recognized the scope of freedom of press as an essential prerequisite of a democratic form of democratic form of government and regarded it as the mother of all other liberties in democratic society.
In the matter of Sahara India Real Estate Corpn. Ltd. v. SEBI [11] the Supreme Court discussed postponement orders i.e., judicial orders restraining the media on reporting regarding matters. This is done with the motive of ensuring proper administration of justice and fairness of trial. Another important aspect highlighted was that even in matters where fair and accurate reporting takes place there is also a real and substantial risk of serious prejudice to connected trials. Also, postponement orders are also a means to avoid contempt. This is for the protection of media lest it commit contempt in its zeal to pursue a story. These orders are also a useful tool to balance conflicting public interests in terms of both safeguarding the sanctity of the judicial process and the right of freedom of speech and expression being exercised by the media. The Supreme Court had another word of caution in the matter of Satish bhushan Bariyar v. State of Maharashtra [12] held that if media trial is a possibility, sentencing by media cannot be ruled out.
IV. Media and their influence in society
The paid news which is given by any political party or any other big organisation easily deviate the media from the real objective and the media being the mirror to the world or being an eye opener, becomes a puppet in the hand of powers. Hence media being working for the people, by the people and of the people become for the sponsor, by the sponsor and of the sponsor. Sometimes these issues give birth to the media trials in which the media proof someone guilty before the judgement of the court.
In the matter of State of Maharashtra v. Rajendra Jawanmal Gandhi [13] the Supreme Court while considering the issue of sentencing observed that a trial by press, electronic media or public agitation is the very antithesis of the rule of law. This may very well lead to miscarriage of justice and therefore, a Judge should guard himself against any such pressure and should strictly be guided by the rules of law. Parties have a constitutional right to have a fair trial in the court of law, by an impartial tribunal, uninfluenced by newspaper dictation or popular Glamour.
In the Sheena Bohra Murder Case, the eyes of media have pierced the personal life of the main accused Indirani Mukherjee which was fully accused by the media. Every aspect of her personal life and character was in public lens of examination via media. There have been numerous instances in which media has conducted trials of an accused and they had been verdict even before the judgement passed by the judiciary.
In 20th century a famous celebrity Fatty Arbuvckle was proved guilty by the media trial but he was proved not guilty by the Hon’ble Court but due to the media trial his entire career and his reputation was against him due to all the wrong media coverage. In the case of Arushi Talwar Murder Case the media has verdict that the murder has been done by her parents Rajesh Talwar and Nupur Talwar, he was not guilty but the media proved him guilty.
The Law Commission in its 200th report, Trial by Media: Free Speech versus Fair Trial Under Criminal Procedure (Amendment to the Contempt of Courts Act, 1971 ), has recommended a law to debar media from reporting anything prejudicial to the rights of the accused from time to arrest to investigation and trial in criminal proceedings. [14]
On November 2006, the former Chief Justice of India Y K Sabharwal expressed his views on media trials as:
According to law a accused is presumed to be innocent till proven guilty in the court of law, and is entitled to be a fair trial. So, it is legitimate to demand that nobody can be allowed to prejudge or prejudice one’s case? Why should judges be swayed by public opinion?
The Supreme Court reiterated that the media and the judiciary are institutions inhabiting separate spheres and their functions do not overlap. One cannot and must not use the other for discharge of its functions. It was observed that media should only engage in acts of journalism and not act as a special agency for the court. The impermissibility of freedom of speech and expression amounting to interference with the administration of justice due to the prejudicial nature of certain media coverage was also highlighted. [15]
Attorney General of India, K.K.Venugopal while appearing in his personal capacity in the 2009 contempt of court case against lawyer Prashant Bhushan, said that the manner in which court news is being reported by media has serious implications [16] has been held to quote “Today electronic and print media are freely commenting on pending cases in an attempt to influence judges and public perception. This is doing great damage to the institution,”.
To conclude, Freedom of press has always been a cherished right in all democratic countries and the press has rightly been described as the Fourth Pillar of Democracy. Media can be regarded as the fourth pillar of democracy until and unless the transparency will be there and in this era the media is considered as the daily necessity because the day starts with the media and ends with the same whether its social media or print media or electronic media. Upon a collective assessment of the judgments of the Supreme Court of India on the aspect of media trial it is clear that the risk that they pose is real. The State and the Fourth Estate have a responsibility to defer to each other’s respective domains. While the State should be circumspect regarding any censorship or penal action against the media, at the same time the media should refrain from any unwarranted transgressions. Media trials entail the possibility of subverting administration of justice right from the stage of investigation, trial and finally sentencing. In today’s age of click-bait journalism aimed at satisfying the increasingly short attention span of viewers there exists a subtle by clearly defined line which should not be crossed. Factual narration in itself is safe, however done with a pre-disposed view towards guilt or innocence without any official indictment is clear case of overreach by the media.
[1] State of Maharashtra v/s Rajendrajawanmal Gandhi., (1997) 8 SCC 386
[2] Nariman, Fali S., Are Impediments to Free Expression in the Interest of Justice, CIJL Yearbook, Vol 4, 1995.
[3] http://docs.manupatra.in/newsline/articles/Upload/0158AEEE-1A16-473C-A41A-DB93A66000EB.pdf
[4] Brij Bhushan v. State of Delhi AIR 1950 SC 129
[5] Romesh Thapar v. State of Madras AIR 1950 SC 124
[6] India Express Newspaper Ltd. v. Union of India AIR 1986 SC 515
[7] LIC v. Manubbai Shah (1992) 3 SCC 637.
[8] Printers (Mysore) Ltd. v. Assistant Commercial Trade Officer1994 SCR (1) 682
[9] R.Rajagopal v. State of Tamil Nadu AIR 1995 SC 264
[10] (1996) 6 SCC 466
[11] Sahara India Real Estate Corpn. Ltd. v. SEBI; (2012) 10 SCC 603
[12] Satish bhushan Bariyar v. State of Maharashtra; (2009) 6 SCC 498
[13] State of Maharashtra v. RajendraJawanmal Gandhi; (1997) 8 SCC 386
[14] http://docs.manupatra.in/newsline/articles/Upload/0158AEEE-1A16-473C-A41A-DB93A66000EB.pdf
[15] R.K. Anand v. Delhi High Court; (2009) 8 SCC 106
SEE ALSO: M.P. Lohia v. State of W.B.; (2005) 2 SCC 686.
[16] https://www.hindustantimes.com/india-news/media-trial-causing-great-damage-to-judiciary-attorney-general-kk-venugopal/story-XXroXLeMrdHYAKP85SjsgL.h
Total number of HTML views: 24869
Total number of pdf downloaded: 1158, open access.
http://doi.one/10.1732/IJLMH.26050
Recent content
1 what are trade sanctions.
By Parvathy Girish and Adam Zamin Sheikh
Volume: 7 Issue : 5 Page: 103 - 116
2 Strategic Mineral Security and its Role in National Security
By Elan Sanjeevi and Anmol Singh
Volume: 7 Issue : 5 Page: 96 - 102
3 Critical Mineral Security and its Role in Greener Technologies
Volume: 7 Issue : 5 Page: 87 - 95
4 Ensuring Dignity in Death: A Case Analysis of Common Cause v. Union of India
By Ankita Rituraj
Volume: 7 Issue : 5 Page: 80 - 86
5 AI and Work: Influence of Automation on the Indian Labour Market
By Madhumitha Shankar
Volume: 7 Issue : 5 Page: 67 - 79
International Journal of Law Management & Humanities
Typically replies within 24 hours.
Any questions related to the journal or your submission?
WhatsApp Us
🟢 We will respond within 24 hours, maybe less.
WhatsApp us.
Media Trial: Role of Media under Indian Constitution
7 Pages Posted: 8 Sep 2021
Dr. Jay Kumar Bhongale
Bharati Vidyapeeth Deemed to be University, New Law College, Pune
Date Written: August 13, 2021
The media’s the most powerful entity on earth. They have the power to make the innocent guilty and to make the guilty innocent, and that’s power. Because they control the minds of the masses.” Malcolm X. This quotation speaks itself the gravity and concrete nature of media in democratic set-up of any nation. Present article will focus on current operational interference of media in administration of justice, the role of Supreme Court and its power under constitution. The unavailability of mindset of legislature to insert the effective statute and negation of Supreme Court to impose guidelines on Media Trials, uncertainty of implementing of Law Commission Guidelines, the outcome of such approach and failure to tie jaws of uncontrolled media by government and Court which harming rule of law in India.
Keywords: Accountability of Media, Contempt of Court, Freedom of Expression, Interference of Media, Prejudicial Publicity, Postponement
Suggested Citation: Suggested Citation
Dr. Jay Kumar Bhongale (Contact Author)
Bharati vidyapeeth deemed to be university, new law college, pune ( email ).
Educational Complex, Erandwane, Paud Road, Pune, 411038 India
Do you have a job opening that you would like to promote on SSRN?
Paper statistics, related ejournals, comparative & non-u.s. constitutional law ejournal.
Subscribe to this fee journal for more curated articles on this topic
India Law eJournal
Law, politics & the media ejournal, criminal law, courts & procedure ejournal, criminology ejournal, communication law & policy ejournal, political economy - development: political institutions ejournal.
- DOI: 10.1080/10811689709368632
- Corpus ID: 144832599
Trial by media?: Media reliance, knowledge of crime and perception of criminal defendants
- J. W. Wright , S. D. Ross
- Published 1 September 1997
- Law, Sociology
- Communication Law and Policy
20 Citations
Murder in black and white, the newspaper coverage of homicide in houston, the impact of pretrial publicity on “eye for an eye” retributivist support and malicious perceptions of criminal offenders.
- Highly Influenced
WRONG SIDE OF THE TRACKS: EXPLORING THE ROLE OF NEWSPAPER COVERAGE OF HOMICIDE IN SOCIALLY CONSTRUCTING DANGEROUS PLACES *
Characteristics of contemporary gag order requests in media law reporter volumes 19 through 33, dingo media the persistence of the “trial by media” frame in popular, media, and academic evaluations of the azaria chamberlain case, monkey business in a kangaroo court: reimagining naruto v. slater as a litigious event, public knowledge and perceptions about unsubmitted and untested sexual assault kits, demented mother, maniac with a gun, madman: prejudicial language use in historical newspaper coverage of multiple-child murders in new zealand, community violence in dar es salaam, tanzania: a mixed methods study, 32 references, pretrial publicity: a field study, media effects on jurors, media use and perceptions of crime, prejudicial publicity: its effect on law school mock juries, the conduct of voir dire: a psychological analysis, the prejudicial impact of pretrial publicity1, pretrial publicity, judicial remedies, and jury bias, cultural indicators: violence profile no. 9., the use of social science data in a change of venue application: a case study, on the effectiveness of voir dire in criminal cases with prejudicial pretrial publicity: an empirical study, related papers.
Showing 1 through 3 of 0 Related Papers
- My Shodhganga
- Receive email updates
- Edit Profile
Shodhganga : a reservoir of Indian theses @ INFLIBNET
- Shodhganga@INFLIBNET
- Chaudhary Charan Singh University
- Department of Law
| Title: | Trial by media and its impact upon judicial trial a critical study |
| Researcher: | Mittal, A K |
| Guide(s): | |
| Keywords: | Social Sciences,Social Sciences General,Law Impact Upon Judicial Trial |
| University: | Chaudhary Charan Singh University |
| Completed Date: | 2016 |
| Abstract: | None newline |
| Pagination: | xx, 279p. |
| URI: | |
| Appears in Departments: | |
| File | Description | Size | Format | |
|---|---|---|---|---|
| Attached File | 244.18 kB | Adobe PDF | ||
| 101.29 kB | Adobe PDF | |||
| 11.98 kB | Adobe PDF | |||
| 14.26 kB | Adobe PDF | |||
| 22.77 kB | Adobe PDF | |||
| 229.12 kB | Adobe PDF | |||
| 2.52 MB | Adobe PDF | |||
| 2.46 MB | Adobe PDF | |||
| 125.6 kB | Adobe PDF | |||
| 106.62 kB | Adobe PDF | |||
| 600.57 kB | Adobe PDF | |||
| 865.07 kB | Adobe PDF | |||
| 1.1 MB | Adobe PDF | |||
| 62.42 kB | Adobe PDF | |||
| 55 kB | Adobe PDF | |||
| 308.03 kB | Adobe PDF |
Items in Shodhganga are licensed under Creative Commons Licence Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).

Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
- We're Hiring!
- Help Center
Trial by Media
- Most Cited Papers
- Most Downloaded Papers
- Newest Papers
- Mediated Scandals Follow Following
- Public Inquiries Follow Following
- Whistleblowing Follow Following
- Corruption Follow Following
- Reform Follow Following
- Trial by media as a legal problem,a comparative analysis Follow Following
- Scandals Follow Following
- Criminology Follow Following
- Property and Human rights Follow Following
- Law and culture Follow Following
Enter the email address you signed up with and we'll email you a reset link.
- Academia.edu Journals
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Neurobiological research on N,N -dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors
- Open access
- Published: 10 September 2024
- Volume 81 , article number 395 , ( 2024 )
Cite this article
You have full access to this open access article

- Klemens Egger ORCID: orcid.org/0000-0001-5072-9674 1 , 2 , 3 ,
- Helena D. Aicher ORCID: orcid.org/0000-0001-5915-7086 1 , 2 , 4 ,
- Paul Cumming ORCID: orcid.org/0000-0002-0257-9621 3 , 5 &
- Milan Scheidegger ORCID: orcid.org/0000-0003-1313-2208 1 , 2
1 Altmetric
The potent hallucinogen N,N- dimethyltryptamine (DMT) has garnered significant interest in recent years due to its profound effects on consciousness and its therapeutic psychopotential. DMT is an integral (but not exclusive) psychoactive alkaloid in the Amazonian plant-based brew ayahuasca, in which admixture of several β -carboline monoamine oxidase A (MAO-A) inhibitors potentiate the activity of oral DMT, while possibly contributing in other respects to the complex psychopharmacology of ayahuasca. Irrespective of the route of administration, DMT alters perception, mood, and cognition, presumably through agonism at serotonin (5-HT) 1A/2A/2C receptors in brain, with additional actions at other receptor types possibly contributing to its overall psychoactive effects. Due to rapid first pass metabolism, DMT is nearly inactive orally, but co-administration with β -carbolines or synthetic MAO-A inhibitors (MAOIs) greatly increase its bioavailability and duration of action. The synergistic effects of DMT and MAOIs in ayahuasca or synthetic formulations may promote neuroplasticity, which presumably underlies their promising therapeutic efficacy in clinical trials for neuropsychiatric disorders, including depression, addiction, and post-traumatic stress disorder. Advances in neuroimaging techniques are elucidating the neural correlates of DMT-induced altered states of consciousness, revealing alterations in brain activity, functional connectivity, and network dynamics. In this comprehensive narrative review, we present a synthesis of current knowledge on the pharmacology and neuroscience of DMT, β -carbolines, and ayahuasca, which should inform future research aiming to harness their full therapeutic potential.
Avoid common mistakes on your manuscript.
Introduction
Because of their profound effects on the human mind, psychedelic substances have been the object of fascination in the Western world since the 1950s [ 1 ], when Humphrey Osmond coined the term psychedelic. Despite pioneering work by Alexander and Ann Shulgin on the synthesis and subjective effects of phenylethylamines and tryptamines [ 2 , 3 ], a long-standing moratorium on funding of psychedelics research impeded progress in understanding basic aspects of the physiology and phenomenology of psychedelic substances. In this narrative review, we summarize the state of knowledge of N,N -dimethyltryptamine (DMT), which has been used for millennia by indigenous peoples of South and Mesoamerica for healing and spiritual purposes in the form of the herbal brew variously known as yajé or ayahuasca [ 4 , 5 ]. Footnote 1 Uniquely, ayahuasca brew often contains a mixture of DMT along with several β -carboline alkaloids, which together enhance the bioavailability of orally administered DMT by blocking its first pass metabolism by monoamine oxidase A (MAO-A) in the gut and other organs. Whereas oral DMT alone is nearly inactive, DMT is potently psychoactive when inhaled as vapor [ 6 ], and when taken via intravenous administration [ 7 , 8 ], i.e., routes that circumvent the first-pass metabolism.
The classical psychedelics lysergic acid- N,N -diethylamide (LSD) [ 9 ] and psilocybin (prodrug of the psychoactive substance psilocin) are agonists or partial agonists at 5-hydroxytryptamine (serotonin) 2A (5-HT 2A ) receptors in brain [ 1 ], which are the key mediators of their psychedelic effects. While DMT is generally included among the classical psychedelics, as shall emerge below, it is not yet certain that 5-HT 2A receptor agonism exclusively mediates DMT/ayahuasca effects. Investigations of ayahuasca’s pharmacological effects and therapeutic potential are at a relatively nascent stage, mainly confined to its use in naturalistic and traditional settings.
Like classical psychedelics, consumption of ayahuasca leads to profound alterations in consciousness, characterized by changes in perception and the “inner (cognitive and emotional) experiences” [ 5 , 10 , 11 ], with “visuals, kaleidoscopic lights, geometrical forms, tunnels, animals, humans and supernatural beings coinciding with sensations of peace, harmony and inner calm” [ 12 ]. Other commonly experienced phenomena include synesthesia [ 13 ], decentered introspective states [ 14 ], emotional release [ 15 ], attribution of meaning [ 14 , 16 ], alterations in meaningful, guiding values in life [ 14 , 17 ], ego dissolution, better understanding of oneself and others, acceptance of oneself and past life events [ 14 , 18 ], and expansive states with transpersonal experiences [ 19 ]. Unlike other psychedelics, ayahuasca effects also include notable physical sensations like nausea and vomiting, which may be integral to its traditional use in healing and spiritual rituals [ 20 ]. Indigenous and neo-shamanic groups attribute transformative healing properties to the spirit of ayahuasca, often experienced through vivid encounters with plant spirits in a culturally rich ritual setting [ 21 ].
Recent pre-clinical and observational studies have shown encouraging results with ayahuasca in treating a variety of conditions and their animal models, including depression, anxiety, PTSD [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ], substance use disorders [ 34 , 35 , 36 , 37 , 38 ], eating disorders [ 39 , 40 ], and grief [ 41 , 42 ]. In an initial clinical trial, ayahuasca has shown efficacy against depression and anxiety symptoms [ 28 ] and in altering brain network dynamics linked to depression pathophysiology [ 43 ]. In a randomized placebo-controlled trial (RCT) conducted in Brazil, a single ayahuasca dose produced rapid antidepressant effects persisting for weeks in (n = 29) patients with treatment-resistant depression [ 30 ]. In a recent observational study, the majority of (n = 20) individuals with initial diagnosis of major depression disorder (MDD) enjoyed remission lasting a year after their participation in a ritual with administration of botanical ayahuasca analogues (i.e., various plant sources of DMT and β -carbolines) in the context of an ayahuasca ritual [ 32 ]. Preclinical and in vitro investigations suggest that ayahuasca chemical constituents may also possess neuroprotective properties in neurodegenerative disease models [ 44 , 45 , 46 ]. Thus, a comprehensive review of 21 clinical and preclinical studies with chemical constituents of ayahuasca revealed consistent findings of anxiolytic, antidepressant, anti-addictive, and neuroprotective properties [ 47 ].
Psychological support is critically important during a therapeutic ayahuasca experience, given the influence of contextual factors on mental health outcomes [ 48 ]. The burgeoning interest in ayahuasca's therapeutic benefits marks a pivotal shift from traditional to clinical contexts, opening new avenues for research and application in Western medicine. Its uniquely complex blend of pharmacological, psychological, and cultural elements makes ayahuasca an intriguing research area for scientists from various disciplines. Our objective in this narrative review is to bridge the gap between the phenomenology of the ayahuasca experience and western models of neuropharmacology and brain function. Therefore, we have compiled the current state of knowledge of the pharmacology, biochemistry, and neuroscience of DMT, emphasizing its synergism with MAOIs in the contexts of ayahuasca and its botanical and synthetic analogs. We first summarize the historical and cultural background of ayahuasca, and then elaborate upon the known pharmacological, molecular, cellular, and functional mechanisms of action of the DMT/MAOI combination from studies in vitro and imaging studies in vivo.
Ayahuasca: traditional botanical forms
Ayahuasca (also known as yajé, hoasca, etc.) is a Hispanicized term borrowed from Quechuan dialects of the Amazon basin, which refers to the woody vine (liana) Banisteriopsis caapi and its decoctions, as used for ritual and healing purposes [ 5 ]. The psychoactive beverage is prepared by extensive boiling of the B. caapi bark, resulting in a thick, brown, and oily liquid [ 49 ]. The prolonged boiling process is necessary to extract the plants’ alkaloids, which have low solubility in water. Indeed, the β -carboline harmine mainly resides in the solid phase of the ayahuasca brew [ 50 ]. Recipes for traditional ayahuasca differ between indigenous peoples and geographic regions [ 51 ]. Some traditional shamanic rituals using ayahuasca as a sacred medicine employ decoctions mainly from B. caapi , which contains β -carboline MAOIs, but little or no DMT. Traditional ayahuasca decoctions often contain DMT derived from the leaves of plants such as Psychotria viridis, P. carthagenensis, or the amazonian shrub Diplopterys cabrerana [ 52 , 53 ] . In popular conception, the B. caapi MAOIs serve only to enhance the bioavailability of DMT derived from other ayahuasca components. However, DMT-containing plants are not always included in ayahuasca brews; some indigenous groups in the Amazon basin use B. caapi alone for initiation or healing practices , without admixture of any other plant material [ 54 , 55 ]. Furthermore, some ayahuasca decoctions contain tobacco or other psychoactive plants [ 56 ]. Nonetheless, we suppose that a binary DMT/MAOI model may best capture the complex ayahuasca experience that derives from ancient traditional knowledge of indigenous people who have used these brews in one form or another since millennia [ 57 ].
The essential ayahuasca component B. caapi contains several β -carbolines from the harmala alkaloid family of tryptophan metabolites [ 58 ], which may be psychoactive in their own right [ 59 ], in addition to their inhibition of DMT metabolism by MAO-A [ 60 ]. The various β -carbolines in B. caapi , especially harmine and harmaline, enable the attainment of sufficient plasma DMT concentrations to evoke psychedelic effects lasting 4–6 h [ 5 , 61 ]. Re-dosing four hours after the first ayahuasca administration prolongs the subjective effects, likely due to accumulation of alkaloid concentrations in the body [ 62 ] (repeated dosing is typical of traditional ayahuasca rituals). Tetrahydroharmine (THH), the second-most abundant B. caapi β -carboline, is also a weak inhibitor of plasma membrane serotonin transporters (SERT) [ 63 ], i.e., the site of action of selective serotonin reuptake inhibitor (SSRI) antidepressants. THH may also contribute to net MAO inhibition despite its weaker affinity as compared to harmine and harmaline [ 10 , 64 ]. The B. caapi β -carbolines are almost exclusively MAO-A inhibitors, with 100-fold lower affinity for MAO-B [ 65 , 66 ]. However, it is by no means certain that DMT and β -carbolines are the only pharmacologically relevant compounds in ayahuasca; the chemical diversity in the plant matrix predicts an “entourage effect” [ 67 ] that remains uninvestigated. For the present, we focus on the most abundant ayahuasca β- carbolines (harmine, THH and harmaline) and their interactions with DMT [ 68 ].

β -carbolines and DMT concentrations in ayahuasca samples
We summarize in Table 1 findings of studies reporting concentrations of harmine, harmaline, THH, and DMT in ayahuasca samples from different geographical and indigenous origins. In considering the results of these field sample studies, there is clearly no standard alkaloid composition or standard dose, and that factors such as quantity and quality of used plants, the geographic region, and likewise the cultural affiliations of the people producing ayahuasca all contribute to its varying composition [ 69 ] . The rank order of β -carboline concentrations is generally harmine ≥ THH > harmaline, where harmine concentrations tended to only slightly exceed the THH concentrations, and harmaline was overall the least-abundant β -carboline alkaloid. Indeed, the reported concentrations range from 0.06 to 22.9 mg/mL harmine, 0–1.72 mg/mL harmaline, 0.02–23.8 mg/mL THH and 0.05–14.2 mg/mL DMT (Table 1 ). Despite considerable variability, the analytical findings generally predict that one cup (200 mL) of typical ayahuasca brew would contain alkaloid doses up to a few hundred mg. During the extended boiling process of ayahuasca preparation, harmine converts via consecutive reduction reactions to harmaline and then to THH, thus shifting the β -carboline ratios as compared to the untreated B. caapi [ 70 ]. Furthermore, THH is more chemically stable than harmine/harmaline, surviving in ayahuasca stored for nine days at 37 °C [ 71 ]. Variable DMT concentrations likely reflect the proportion of P. viridis to the total plant material, which ranged from 7 to 20%, depending on the preparation recipe [ 70 ]. It remains unknown if alkaloid concentrations in B. caapi differ across geographic regions or depending on season of harvest.
Ayahuasca analogues and pharmahuasca
The eponymic harmala β -carboline alkaloids in B. caapi also occur in plants such as Peganum harmala (Syrian rue), which is native to Eurasia and northern Africa, or the flowers of the mainly American Passiflora incarnata (passionflower). DMT in ayahuasca often derives from plants of genera Psychotria , the Brazilian/Mesoamerican Mimosa hostilis (jurema), or Anadenanthera and Diplopterys [ 53 , 77 ]. The ubiquity of these alkaloids likely reflects their derivation from the amino acid tryptophan, but there is evidence that tryptamine alkaloids confer increased resistance against herbivores or other predators [ 78 , 79 ]. Brews comprising plant sources other than B. caapi and Psychotria are ayahuasca analogues, whereas synthetic formulations are commonly known as “pharmahuasca” [ 53 , 56 ]. Ayahuasca analogue formulations commonly include P. harmala as a β -carboline source and M. hostilis or A. confusa as a DMT source [ 53 , 56 , 80 ]. P. harmala (mainly its seeds) has traditional medicinal uses in Iran [ 81 ] for its supposed cardiovascular, neurologic, antimicrobial, gastrointestinal (GI), and antidiabetic effects [ 82 ], and M. hostilis finds use in South and meso-American spiritual and shamanic rituals [ 83 , 84 ].
In the 1960s, Claudio Naranjo reported on the use of harmaline for Western psychotherapy [ 85 ], highlighting its potential therapeutic benefits for facilitating introspection, emotional release, self-awareness, and personality integration. It remains uncertain if such effects derive from MAOI or other pharmacological properties of harmaline. Advancements in DMT synthesis and the broader availability of pharmaceutical MAOIs were drivers for the increasing popularity of pharmahuasca. Particularly in Europe, ayahuasca analogues and pharmahuasca are often less costly and more accessible than authentic ayahuasca [ 53 , 86 , 87 ]. Furthermore, uncontrolled harvesting of B. caapi is a recognized threat to its viability in the wild [ 88 ]. While ayahuasca analogues and pharmahuasca can produce experiences akin to traditional ayahuasca, their specific effects differ according to the alkaloid composition [ 86 , 87 ]. Synthetic formulations potentially offer more standard alkaloid composition and a better safety profile, notably with respect to the occurrence of emesis (a”purge” is considered an essential and therapeutic aspect of the ayahuasca ritual) [ 89 ]. Indeed, having a standard composition remains a key requirement for inclusion of medicine in an approved Western pharmacopeia, although there is not yet a consensus on the optimal composition of ayahuasca alkaloids.
N,N- dimethyltryptamine (DMT)
DMT derives from tryptamine, which forms by decarboxylation of L -tryptophan catalyzed by the enzyme aromatic amino acid decarboxylase (AAADC; commonly known as DOPA decarboxylase) (Fig. 1 ). As first described by Axelrod [ 90 ], DMT biosynthesis proceeds by a two-step process from tryptamine via the enzyme indolethylamine N -methyltransferase (INMT), a transmethylation enzyme using S -adenosyl- L -methionine (SAM) as methyl donor. The product N -methyltryptamine (NMT) undergoes further methylation by the same enzyme to give DMT. In situ hybridization studies revealed expression of INMT in neurons, co-localizing with DOPA decarboxylase in presumably DMT-synthesizing neurons in cerebral cortex, and in choroid plexus, but with highest concentration in lung tissue [ 91 , 92 ]. However, INMT knockout in a rodent model, failed to ablate tryptamine methylation in brain and lung tissue, suggesting the presence of alternate enzymatic pathways [ 93 ]. DMT is present in many mammalian tissues. Indeed, the interstitial DMT concentration in rodent brain was approximately 1 nM to cerebral microdialysis coupled with HPLC [ 92 ]. Cerebral microdialysis analysis of canonical biogenic monoamine neurotransmitter concentrations (e.g., serotonin, dopamine, norepinephrine) showed similar concentrations in the range of ~ 1–4 nM [ 94 ]. UHPLC-MS analysis of brain tissue extracts indicated DMT concentrations ranging from zero to 30–60 nM [ 95 , 96 ]. The detection of DMT in the pineal gland [ 97 ] inspired the concept that pineal DMT release might induce vivid dreams, or near-death and other mystical-type experiences [ 98 ], but the total quantity of pineal DMT seems insufficient to evoke such effects. Studies of endogenous DMT concentrations in body fluids (mainly blood and urine) are generally uninformative about the cellular sites of DMT production in biologically significant amounts [ 98 ].
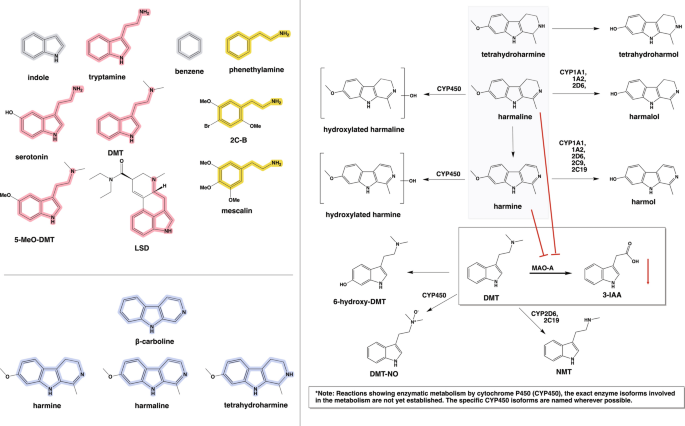
Molecular structures of N,N- dimethlytryptamine (DMT) and other psychedelics, the main ayahuasca β -carbolines, and key metabolic pathways. A Indole and benzene rings (gray) are the chemical scaffolds of the two main categories of psychedelics, i.e. tryptamines (red) and phenethylamines (yellow). Serotonin, DMT and 5-MeO-DMT are structurally similar; LSD, while also containing the tryptamine (and phenethylamine) motif, is an ergoline derivative. Among the phenethylamine psychedelics, we present the structures of 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and mescaline. B The β -carboline scaffold of harmine, harmaline, and tetrahydroharmine (THH) are shown in blue. C) These main β -carbolines in ayahuasca undergo demethylation to harmol, tetrahydroharmalol, and harmalol, respectively. Several cytochrome (CYP) enzymes are implicated in the demethylation of harmine and harmaline, but details are lacking for THH. Harmine and harmaline can also undergo ring-hydroxylation catalyzed by CYP450 [ 107 , 108 ]. An additional metabolic route of harmaline is its oxidation to harmine. DMT is predominantly metabolized by oxidative deamination via monoamine oxidase type A (MAO-A), followed by formation of indole-3-acetic acid (3-IAA) by non-specific aldehyde dehydrogenases. Alternately, DMT is oxidised to DMT- N -oxide (DMT-NO) by CYP450 or demethylated by CYP2D6 and CYP2C19 to N- methyltryptamine (NMT), or hydroxylated to 6-hydroxy-DMT by yet unknown enzymes [ 60 , 107 , 109 , 110 ]. Red arrows indicate inhibition of DMT metabolism by the β -carboline MAO-A inhibitors, resulting in lesser formation of 3-IAA
Exogenous DMT rapidly accumulates in the rat brain after i.p. or i.v. administration, transiently attaining a brain:blood partition ratio of approx. 5–6:1, followed by rapid clearance from the brain and circulation [ 96 , 99 , 100 , 101 ]. Nonetheless, DMT remained detectable in the rabbit CNS up to seven days after peripheral administration, while urinary excretion was not detectable after 24 h [ 102 ], which could be consistent with storage in a very stable vesicular pool. After i.p. administration, there was DMT accumulation in the cerebral cortex, amygdala, and caudate-putamen, while medulla oblongata and cerebellum only showed low uptake [ 101 ], suggesting compartmentation within specific neuronal populations. We have reported spatially heterogeneous DMT accumulation in rat brain after i.p. administration, with 50% higher concentrations in the frontal cortex than in the cerebellum [ 103 ], again suggesting some mechanism for its retention in brain tissue. Indeed, DMT can enter serotonin neurons via SERT, and then accumulate in synaptic vesicles as a substrate for the vesicular monoamine transporter 2 (VMAT2) [ 104 ]. Storage in a vesicular compartment would protect DMT from MAO degradation, and might support its release from serotonin fibers as a “false neurotransmitter” [ 101 ]. To qualify as a classical neurotransmitter, an endogenous substance must be present in physiologically significant amounts, with release in a calcium-dependent manner after presynaptic depolarization, and then evoking responses at specific post-synaptic sites [ 105 ]. Given the current evidence, endogenous DMT may meet these criteria [ 106 ], despite its low affinity at 5-HT 2A receptors. For an extensive discussion of DMT as a candidate neurotransmitter, see [ 106 ].
MAO inhibitors
MAO enzymes (enzyme commission number EC 1.4.3.4) are amine oxidoreductases, with main expression in the outer mitochondrial membrane of mammalian cells. MAO substrates include the biogenic monoamine neurotransmitters dopamine, epinephrine, norepinephrine, and serotonin, and the exogenous psychedelics DMT, psilocin and mescaline. The MAO-reaction consumes molecular oxygen in the restoration of the reduced FADH 2 cofactor to its active FAD form; the imine intermediate spontaneously eliminates ammonia, and the resultant aldehyde is oxidised to the carboxylic acid by non-specific NAD + -dependent dehydrogenase enzymes [ 111 ]. The two isoforms of MAO, which arose from a gene duplication event, have very similar amino acid sequences [ 112 ], but somewhat distinct primary substrates. Whereas serotonin and DMT are preferred substrates for MAO-A, phenylethylamine is a MAO-B substrate; both isozymes metabolize dopamine and tyramine with little selectivity [ 113 , 114 ]. MAO-A occurs in the brain, GI tract, liver, the vasculature of the lungs, as well as in the placenta, while MAO-B mainly occurs in blood platelets [ 111 ], astrocytes [ 115 ], and certain specific populations of neurons [ 116 ]. With respect to ayahuasca, MAO-A in the GI tract is the principal determinant of DMT absorption.
Whereas harmine and moclobemide are reversible MAO-A inhibitors, certain propargyl compounds form a covalent bond with the enzyme, rendering it permanently inactive. The non-selective irreversible MAOIs phenelzine, isocarbaxazid, and tranylcypromine emerged in the mid-twentieth century as the first effective pharmacotherapeutic agents for depression [ 117 ]. These medications have since largely fallen out of favor due to the perceived risk of interactions with dietary vasoactive amines (the”cheese effect”) or the serotonin syndrome, a potentially fatal crisis of hypertension, fever, delirium, and rhabdomyolysis that can occur upon co-administration of direct or indirect serotonin agonists. As such, irreversible MAOIs now seldom serve as first or second line antidepressants, but remain in use in certain severe and treatment-resistant cases, which calls for strict observation of dietary restrictions [ 118 ]. However, serotonin syndrome and hypertensive crisis are exceedingly rare events in patients treated with irreversible MAO blockers [ 118 ].
The reversible MAO-A inhibitor moclobemide is an antidepressant with some efficacy in treating social anxiety, being notable for its favorable side-effect profile and relatively brief plasma half-life. Moclobemide has occasionally been detected in neo-shamanic recipes in Europe [ 53 ]. In general, pretreatment with any inhibitor of MAO-A, reversible or irreversible, would likely serve for potentiation of DMT bioavailability after oral administration, we are not aware of MAOIs other than harmine and moclobemide finding use in pharmahuasca.
Safety and risks associated with ayahuasca or DMT use
Despite the theoretical risk of serotonin syndrome, there are preclinical reports showing potentiation of DMT effects by co-administration of irreversible MAO inhibitors iproniazid or pargyline treatment [ 119 , 120 ]. In an ayahuasca neurotoxicity study, some rats showed behavioral signs of serotonin syndrome and eventually died after receiving doses some 30- and 50-fold the typical human doses [ 121 ]. However, only at such extreme doses can the reversible MAOIs in ayahuasca (or generally also pharmahuasca) evoke the nearly complete inhibition that may be a precondition for the serotonin syndrome. Observational studies have not raised major safety concerns for ayahuasca practitioners taking SSRIs [ 122 ].
Neither short-term nor long-term ayahuasca use led to dependency, and its use in controlled settings such as ceremonial contexts suggests an acceptable safety profile [ 49 , 76 , 123 ]. Acute treatment-emergent adverse events (TEAEs), mainly nausea and vomiting (69.9%), typically resolved without an intervention, with few (2.3%) such participants needing medical attention [ 124 , 125 ]. The American National Poison Data System (NPDS) registered 538 adverse events for ayahuasca between 2005 and 2015, with 28 cases requiring intubation, four cases of cardiac arrest, 12 seizures, and three fatalities [ 126 ]. When considering the global prevalence of ayahuasca use, estimated to be over 4 million annually, the number of deaths (n = 58) reported in association with its use is low. Notably, those fatalities have not been linked to traditional ayahuasca ingredients but may involve toxic plant admixtures, drug interactions, or pre-existing conditions [ 127 ].
On the other hand, challenging psychedelic experiences are common (55.9%), with adverse psychological reactions typically subsiding within a few days; however, 12% of such individuals sought additional professional support [ 124 , 125 ]. Severe psychological distress, including severe depression and psychotic episodes, can occur with ayahuasca use [ 128 , 129 ]. Contemporary neo-shamanic and tourist-oriented settings therefore adopted a broad spectrum of general safety and good practice guidelines. However, some participants in contemporary ayahuasca rituals may lack adequate cultural support and guidance [ 129 , 131 ]. Traditional indigenous settings usually provide structure and safety within ancestral medicinal practices (e.g. plant dietas) contemporary touristic settings. While certain structured approaches like specific dietary protocols, careful attendance, and setting might mitigate risks and enhance the experience [ 130 ], the Western concept of psychological support may not neatly align with such Indigenous methods. Traditional indigenous settings often lack formal health screenings and discussions on medication interactions, challenging the assumption that they are inherently safer for tourists.
Importantly, there is need to integrate safety measures for interactions between ayahuasca with prescription medications (i.e., SSRIs or dopaminergic stimulants), other drugs of abuse, or specific foods rich in tyramines such as overripe fruits, fermented food, tofu, or nuts, which might conceivably increase the risk of serotonin syndrome [ 11 , 128 ]. The use of ayahuasca is not recommended for individuals with uncontrolled hypertension, cardiovascular or cerebrovascular diseases, epilepsy, glaucoma, and liver or gastrointestinal diseases (e.g. ulcers or gastritis), and during pregnancy [ 131 ]. Furthermore, ayahuasca may be risky for individuals with severe psychiatric conditions, including bipolar or psychotic disorders [ 131 ].
Mechanisms of action: ayahuasca and DMT alone
Pharmacological mechanisms, human pharmacokinetics and pharmacodynamics of dmt and ayahuasca.
In the 1950s, the Hungarian chemist and psychiatrist Stephen Szára undertook the first investigations of psychological and hallucinogenic effects of DMT, which he self-administered intramuscularly (i.m.) as an extract from M. hostilis [ 132 ]. In the 1970s, Dittrich, Bickel, and colleagues presented the first systematic psychological investigations of i.m. DMT administration [ 133 , 134 ]. Rick Strassmann reported that intravenous (i.v.) DMT at doses ranging from 0.03 to 0.25 mg/kg DMT freebase (as fumarate) induced peak psychedelic effects at five minutes for the 0.25 mg/kg dose, with plasma DMT concentrations peaking at 16 ng/mL (85 nM) [ 135 ]. Subjective effects returned to baseline by 30 min. Recent studies tested i.v. DMT with different administration regimens. Such protocols entailed 0–19.2 mg bolus 0.5–0.8 mg/min constant infusion of DMT freebase (as hemifumarate) for up to 90 min (Basel) [ 7 ], 11.2 mg bolus 1.2 mg/min infusion of DMT freebase (as fumarate) for up to 30 min (London) [ 8 ], and constant infusion totaling 13.4 mg DMT freebase (as fumarate) over 10 min (London) [ 110 ]). The Basel study showed dose-dependent increases in heart rate up to 119 BPM and blood pressure up to 159/98 mmHg [ 7 ], peaking shortly after the bolus administration and stabilizing within 10–15 min. This aligns with findings from the London study [ 8 ], suggesting a good physiological safety margin in individuals without cardiovascular disease or hypertension. In the first randomized controlled trial of a standardized ayahuasca-analogue formulation containing DMT/harmine, oral doses included up to 38.4 mg DMT freebase (as hemifumarate) and 250 mg harmine, or up to 69.1 mg intranasal DMT freebase (as hemifumarate) [ 89 ]. DMT was given in 7.7 mg portions at 15 min intervals intranasally, in combination with buccal harmine (up to 200 mg). Autonomic parameters increased transiently after DMT administration and returned to baseline within 120–180 min, with fewer side-effects (e.g. nausea, headache) compared with botanical ayahuasca. In recent intravenous DMT studies, peak plasma concentrations (C max ) were 61 ng/mL at T max (2.9 min) [ 7 ], 32 ng/mL after 11.2 mg DMT bolus followed by 1.2 mg/min [ 8 ], and 63 ng/mL after constant infusion of 1.34 mg/min DMT (freebase weight) [ 110 ]. These C max values correspond to DMT concentration range of 170–335 nM, with apparent plasma half-life (t 1/2 ) of 5–12 min [ 8 , 109 ]. In comparison, C max for intranasal DMT (combined with buccal harmine) was 33 ng/mL at 130–140 min after first administration of the highest dose combination [ 89 ]. Surprisingly, the intravenous DMT studies revealed large inter-individual variability in plasma concentrations [ 7 , 110 ]. This is likely due to individual differences in whole body MAO activity, suggesting a need for personalized dosing. The intranasal/buccal routes of administration considerably improved upon the PK variability of combined oral DMT/harmine [ 89 ]. However, determining the appropriate extent of MAO inhibition to optimize DMT bioavailabilty, remains challenging due to inter-individual differences in harmine metabolism (i.e. rapid vs. slow metabolizers [ 136 ]). Overall, i.v. DMT and parenteral DMT/harmine administration routes can evoke subjective states of controlled intensity and duration, but further refinement of dosing protocols is needed.
The pharmacokinetics of ayahuasca decoctions, which contain a mixture of β-carboline alkaloids, are more complex than for pharmaceutical combinations of DMT and harmine. The presence of THH and harmaline also influence the pharmacodynamics of DMT, while possibly having psychoactive effects unrelated to MAO inhibition. Administration of natural ayahuasca at doses corresponding to 1.4 mg/kg DMT, 4.6 mg/kg harmine, 0.75 mg/kg harmaline and 5.4 mg/kg THH evoked C max values of 25 ng/mL DMT and 110 ng/mL harmine [ 137 ], which are comparable with C max values from the highest dose in the DMT/harmine PK study [ 89 ]. We suppose that THH, given its C max of 329 ng/mL (1.5 µM) from natural ayahuasca, could well contribute to ayahuasca psychopharmacology. An earlier study with administration of lyophilized ayahuasca capsules reported significant plasma concentrations of DMT and THH, but no detectable harmine and harmaline, despite their presence in the capsules [ 61 ]. Indeed, the concentrations of DMT and THH were lower than expected by the authors, based on the ayahuasca PK study conducted earlier by Callaway and colleagues [ 10 ]. The authors interpreted the disparate plasma results as reflecting differing bioavailability of alkaloids in the lyophilized capsules as compared to the botanical ayahuasca brew [ 61 ]. In another study involving administration of two successive ayahuasca doses at four hours apart, there was substantial potentiation of DMT plasma concentrations (approximately 25% higher C max after the second dose) and subjective effects after the second dose [ 62 ]. These results suggest a lack of acute tolerance to subjective effects, and furthermore indicate that carryover of alkaloids from the first dose augments the MAO inhibition from the second dose, which is consistent with the 3–5 h plasma half-lives of harmine and harmaline (40 mg/kg, i.p.) seen in rats [ 138 ]. Indeed, repeated dosing schemes are very common in the ayahuasca ritual, with (anecdotally) little or no development of tolerance on a time scale of days. Such a lacking rapid tolerance development contrasts with LSD or psilocybin, which show significantly declining subjective effects when taken on consecutive days, in association with cross-tolerance [ 139 , 140 ]. On the other hand, the continuous i.v. DMT administration studies reported the strongest subjective effects directly after onset, which subsequently declined despite increasing blood plasma DMT levels over time [ 7 , 8 ]. Such results imply the occurrence of partial acute short-term tolerance to DMT alone, even though there is a general correspondence between pharmacodynamic subjective effects induced by DMT and ayahuasca with the plasma concentrations of the relevant alkaloids. This holds especially well for plasma DMT curves, which are in good accord with the T max for overall intensity, visual effects, side effects, and other subjective acute effects [ 8 , 10 , 61 , 89 , 110 , 137 , 141 ].
Metabolism of ayahuasca alkaloids
The metabolic pathways for DMT and the β -carbolines in ayahuasca are well understood (Fig. 1 ). In additional to the extensive first pass metabolism or oral DMT, there is also rapid second pass oxidative deamination via MAO-A in brain [ 103 ] and other tissues, irrespective of the route of administration. After oxidative deamination, the second-most important metabolic route for DMT is to DMT- N -oxide (DMT-NO) via unspecified hepatic cytochrome P450 (CYP450) enzymes, with minor routes resulting in the production of N -methyltryptamine (NMT) or 6-hydroxy-DMT (Fig. 1 ). Of these metabolites, the former compound is anecdotally psychoactive, according to Shulgin [ 3 ]. Recent studies indicate that the CPY2D6 and CYP2C19 cytochrome oxidase isoforms can contribute to the formation of NMT from DMT [ 109 , 110 ]. However, the specific isoform/s responsible for the conversion of DMT to 6-hydroxy-DMT remain unknown. Inhibition of MAO-A, by reducing or slowing the production of 3-IAA, shifts the branching ratio in favor of the secondary metabolic pathways. Thus, MAO inhibition augments the formation of DMT-NO, NMT, and 6-hydroxy-DMT [ 107 ]. In a rat study with DMT administration alone (1 mg/kg i.p.), the brain concentration of 3-IAA at 100 min was ~ 50-fold higher than that of unmetabolized DMT. However, with co-administration of harmine (1 mg/kg i.p.), the brain exogenous alkaloid concentrations were 34% DMT, 65% 3-IAA, and 1% DMT-NO [ 103 ]. Thus, even with substantial (but incomplete) MAO inhibition, 3-IAA remained the main metabolite in brain. In an analysis of 24-h urine samples collected after ayahuasca administration, there was 1% recovery of unchanged DMT, versus 55% as 3-IAA and 12% as DMT-NO [ 108 ], suggesting that DMT-NO formation may be more important systemically than in brain (DMT-NO is unlikely to cross the blood–brain-barrier). In another urine analysis study, there was 97% excretion of the DMT dose as 3-IAA and 3% as DMT-NO after oral administration [ 6 ]. In contrast, that study showed significantly higher generation of DMT-NO (28%) after smoking, with 63% excreted as 3-IAA and 10% leaving the body unchanged. Despite the lacking MAO-A inhibition in that study, renal elimination as DMT-NO exceeded that seen after ayahuasca administration.
Harmine and harmaline are metabolized in the body to hydroxy-harmine or and hydroxy-harmaline by enzymes from the CYP450 family, or to harmol and harmalol [ 107 ]. Similarly to harmine and harmaline, THH is preponderantly metabolized to tetrahydroharmol [ 107 , 108 ], but the responsible enzymes remain to be established. In 24-h urine samples collected after ayahuasca administration, there were low total recoveries of harmine, THH, and their metabolites as compared to DMT and harmaline recovery, which comprised approximately two-thirds of the administered dose [ 108 ].
Molecular and cellular mechanisms
Molecular targets of dmt and ayahuasca.
Conventional understanding links the psychedelic properties of DMT (and ayahuasca) to agonism at brain serotonin 5-HT 2A receptors [ 52 ]. However, DMT has only modest affinity at these receptors in vitro (K i = 127–1200 nM and IC 50 = 75–360 nM) [ 142 , 143 , 144 , 145 , 146 ]. Additional binding at serotonin 5-HT 1A (K i = 183 nM, IC 50 = 170 nM) and 5-HT 2C receptors (K i = 360–2630 nM, IC 50 = 360 nM), along with other receptor subtypes, have been proposed to contribute to the overall psychoactive effects of DMT [ 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 ]. 5-HT 1A receptors predominantly occur in the limbic system and brain regions that receive projections from other parts of the limbic system, such as the amygdala, hippocampus, cingulate cortex, and certain other neocortex regions [ 151 , 152 ]. In these regions, the 5-HT 1A receptors have post-synaptic localization, while 5-HT 1A receptors in the raphe nuclei are pharmacologically distinct autoreceptor sites that control serotonin release and firing rate [ 153 ]. The 5-HT 1A receptors are mechanistically relevant for the biological understanding of depression [ 151 , 152 ], as 5-HT 1A agonism proposedly improves stress resilience [ 154 ], and modulates HPA axis functioning [ 155 ] and neuroplasticity [ 156 ]. Not only DMT, but also the ayahuasca β- carbolines influence serotonin neurotransmission, either directly (DMT as a 5-HT 1A/2A/2C agonist) or indirectly (THH as a SERT blocker and weak MAO-A inhibitor, and harmine and harmaline as potent MAO-A inhibitors), which could relate to reported anti-depressant effects of ayahuasca [ 157 ]. Interestingly, the co-administration of the 5-HT 1A/1B receptor partial agonist pindolol potentiated the subjective effects of DMT in a human trial [ 135 ], suggesting an autoreceptor regulation of the post-synaptic effects of DMT.
5-HT 2A receptors have highest expression in brain in layer 5 pyramidal neurons in the neocortex, but also occur in limbic and basal brain structures [ 154 ]. As noted above, DMT shows moderate affinity towards 5-HT 2A sites, as does harmine (K i = 230 nM), whereas harmaline and THH show very low 5-HT 2A affinities of 7.8 and > 10 µM, respectively [ 158 , 159 ]. Νotably, pre-administration of the serotonin 5-HT 2A/C blocker ketanserin (as tartrate, 40 mg) significantly diminished (but did not ablate) the neurophysiological and subjective effects of ayahuasca reported by participants via the hallucinogen rating scale (HRS) and the altered states of consciousness (ASC) questionnaire [ 141 ]. There were significant reductions in the HRS subscales affect, perception and intensity, and in the ASC subscale “visionary restructuralization” upon ketanserin pretreatment. However, these subscale scores were still significantly higher than on study days without ayahuasca administration, which implies that 5-HT 2A receptors may not be the solitary site of DMT action. There was no significant ex vivo occupancy by DMT plus harmine (1 mg/kg, each) at rat cortical 5-HT 2A receptors labelled with [ 3 H]ketanserin [ 103 ], a close analogue of the PET ligand [ 18 F]altanserin [ 160 ]). In the rat study higher doses of DMT plus harmine (3 mg/kg, each) also evoked no detectable occupancy at binding sites for [ 18 F]MHMZ, a 5-HT 2A antagonist PET ligand with higher selectivity and binding signal than [ 18 F]altanserin/[ 3 H]ketanserin. Those negative results may call into question the contention that DMT acts exclusively via serotonin 5-HT 2A receptors. In another study, administration to rats of ayahuasca at doses containing 0.3 mg/kg DMT led to extinction of contextual freezing behavior [ 161 ]. With repeated ayahuasca doses, the co-administration of the 5-HT 2A receptor antagonist MDL-11,939 or the 5-HT 1A receptor antagonist WAY-100635 in the limbic cortex blocked the fear extinction effects, again suggesting an action at both receptor types [ 161 ]. The 5-HT 2C receptors have expression in epithelial cells in the choroid plexus and GABAergic neurons in prelimbic prefrontal cortex (PFC), and in other cortical, limbic, and basal ganglia regions, where they may present targets for various neuropsychiatric disorders [ 162 ]. DMT and harmine both show low affinity to 5-HT 2C receptors [ 158 ], but we cannot presently exclude an action of ayahuasca at these sites.
While LSD interacts at dopamine D 2/3 receptors in vitro [ 143 , 163 ] and in vivo [ 164 ], DMT has little affinity at dopamine receptors [ 128 ]. However, the indisputable involvement of brain dopamine in affective disorders, reward learning, and avoidance behaviors in relation to anhedonia [ 165 , 166 ], we may infer an indirect action of ayahuasca at dopaminergic pathways. While ayahuasca β -carbolines likewise have little affinity for dopamine receptors [ 167 ], they may yet mediate indirect effects on brain dopamine via MAO-A inhibition [ 157 ]. Thus, for example, ayahuasca administration increased the dopamine concentration in amygdala of rats [ 168 ]. Nonetheless, as noted above, complete blockade of both forms of MAO did not potentiate the amphetamine-evoked dopamine release in the [ 11 C]raclopride PET competition paradigm [ 169 , 170 ]. On the other hand, local application of harmine (300 nM) substantially increased the electrically evoked release of dopamine in nucleus accumbens brain slices, in a manner seemingly unrelated to MAO inhibition, but apparently involving 5-HT 2A receptors [ 171 ]. Harmine may inhibit dopamine reuptake via DAT [ 107 ] and may somehow contribute to the normalization of aberrant DAT membrane trafficking and DA reuptake rate in addictive disorders [ 172 , 173 ]. Sigma-1 receptors, which are abundant throughout the CNS [ 157 , 174 ], are another potential site of DMT action. However, the reported affinities for DMT towards sigma-1 receptors (K D = 14 µM [ 174 ], K i = 5.2–15.1 µM [ 143 , 175 ]) may not suffice to impart significant effects. Nonetheless, DMT induced reductions in electrophysiological measures (spreading depolarization), which were normalized by co-administration of sigma-1 antagonists NE-100 and asenapine [ 175 ]. The selective sigma-1 receptor agonist PRE-048 evoked a similar reduction in spreading depolarization. Additional immunohistochemistry results in the same study indicate that DMT might have neuroprotective properties against hypoxia or ischemic stroke [ 175 ].
The β -carbolines harmine and harmaline are antagonists at alpha-1 adrenergic receptors, with IC 50 values in the range 31–36 µM [ 176 ], and may inhibit acetylcholinesterase, which would thereby potentiate cholinergic neurotransmission [ 157 ]. Other possible actions of harmine include modulation of GABAergic neuronal transmission [ 177 ] and inhibition of intracellular protein aggregation (perhaps relevant in neurodegeneration models) [ 178 ], which may call for further investigation of therapeutic mechanisms [ 157 ]. Harmine exerts anti-inflammatory, neuroprotective, antidiabetic, and antitumor effects in various models [ 179 , 180 , 181 , 182 ]. Overall, the ayahuasca β -carbolines may have effects extending beyond simple MAO-A inhibition, but with uncertain relevance to ayahuasca psychopharmacology.
Neuroplasticity induced by DMT and β -carbolines
Recent research addresses the possibility that psychedelic substances can induce or reinstate neuroplasticity, e.g., by altering gene and protein expression, post-translational processes, synapse formation, or neurogenesis. While most such studies have concerned psilocybin, there are a few reports on neuroplastic effects of DMT and the ayahuasca β -carbolines (for review, see [ 183 ]. Especially in human research, plasma levels of brain-derived neurotrophic factor (BDNF), a neurotrophin known to regulate synaptic plasticity and neuronal growth [ 184 ], have served as a marker for potential effects of neurogenesis in the context of antidepressant treatment [ 185 ]. While one study showing increased plasma BDNF levels after ayahuasca intake by healthy and depressed individuals [ 186 ], other studies with ayahuasca or DMT showed no significant changes [ 7 , 187 ]. In a preclinical study, there was likewise no increase in plasma BDNF after DMT administration. However, co-treatment with an antagonist of tropomyosin receptor kinase B (TrkB, the high affinity receptor for BDNF), or with an inhibitor of downstream target of TrkB signaling (mTOR), completely blocked the neuroplastic effects of DMT, suggesting significant engagement of the BDNF signaling pathway in mediating neuroplasticity [ 188 ]. In that same study, a single treatment i.p. with DMT (10 mg/kg as free base) increased dendritic spine density and neuronal excitability in PFC neurons, which might explain the antidepressant and fear extinction effects reported in another rat study with DMT [ 189 ]. Increased dendritic spine growth was observed after activation of intracellular 5-HT 2A receptors with DMT, psilocin or psilocybin. These intracellular receptors are mostly inaccessible by endogenous serotonin, thus suggesting that DMT might induce neuroplasticity via an intracellular mechanism, possibly also at the low endogenous concentrations [ 190 ]. Chronic microdosing (0.77 mg/kg DMT freebase (as hemifumarate) 2–3 times per week for 7 weeks) did not alter BDNF levels or 5-HT 2A receptor expression in rats, but nonetheless exerted antidepressant-like behavioral effects and improved fear extinction learning without other seemingly negative behavioral changes [ 191 ]. Interestingly, the authors also reported retraction of dendritic spines in the PFC, but only in female DMT-treated rats. These latter effects may raise concern about the possibility of unfavorable effects with excessive or prolonged microdosing regimens [ 191 ]. Many of the presented findings potentially link to biomolecular underpinnings of affective disorders, e.g. decreased BDNF levels or TrkB signaling could underly depression, or neuroinflammation due to immunological hyperactivity could mediate anxiety symptomatology [ 25 , 185 , 192 , 193 ]. DMT treatment enhanced performance in memory tests and spatial learning in adult mice, while promoting neurogenesis in the subgranular zone of the hippocampus in vitro (tested after 7 days) and in vivo (2 mg/kg repeated doses of DMT either daily over 4 days, or every other day for 21 days) [ 194 ]. Co-administration of a sigma-1 receptor antagonist blocked these effects, which may belie the low affinity reported for DMT at that binding site.
Preclinical studies have implicated harmine as an enhancer of BDNF signaling in rat hippocampus, in association with antidepressant-like effects in a behavioral assay, both for acute and chronic administrations [ 25 , 193 ]. However, other rat studies showed that a high dose of harmine (15 mg/kg as harmine hydrochloride) induced anhedonia in the sucrose preference test, and reduced locomotor activity, without increasing hippocampal BDNF levels [ 195 ]. All three main β -carboline alkaloids in B. caapi promoted neurogenesis in an in vitro assay with progenitor cells from the subventricular and subgranular zone, which are the main niches of adult neurogenesis in mice. Harmine, harmaline and THH all significantly increased stem cell proliferation, migration, and eventual differentiation into neurons to assays in vitro [ 196 ]. Complementing these findings, earlier studies in chick embryo cells [ 197 ] and human neural progenitor cells [ 44 ] showed that harmine (2–5 µM in chick embryo and 7.5–22.5 µM in human progenitor cells) increased mitosis rates. In a mouse model of anxiety, harmine (20 mg/kg i.p. daily for 7 days) reduced anxiety-like behavioral effects and blunted neuroinflammation in the basolateral amygdala [ 192 ].
We emphasize that some studies have reported adverse effects from very high or repeated doses of DMT or ayahuasca [ 121 , 191 , 195 ], in keeping with Paracelsus’ dictum dosis sola facit venenum (only the dose makes the poison). As with any medication, exceeding some therapeutic dose range may offset beneficial effects of appropriate dosage regimens. The involvement of BDNF signaling in the effects of DMT/ayahuasca seem relevant to the association of BDNF with models of depression and anxiety disorders arising from a hyperactive immune system and chronic low-grade inflammation [ 25 , 185 , 192 , 193 ]. As substantiated by the burgeoning publications on neuroplasticity in the psychedelics literature [ 183 ], there is growing interest in the basic biological mechanisms of action of psychedelic substances. A simple model in which DMT and other ayahuasca constituents act exclusively at serotonin 5-HT 2A receptors falls short of explaining the full spectrum of acute and chronic effects.
Functional mechanisms—human brain imaging and EEG studies
We now give a narrative account of the available molecular imaging, fMRI, and EEG studies reporting effects of ayahuasca (14) or DMT (9) on human brain function. We present the studies in chronological order in Supplementary Table 1, including a brief description of the study design, sample, and interventions, along with key results, with a more detailed discussion in the following section.
Neuroimaging studies with ayahuasca and DMT
The first ayahuasca neuroimaging study used single photon emission tomography (SPECT) to determine the acute effects of lyophilized ayahuasca capsules on regional cerebral blood flow (CBF) [ 198 ], a surrogate marker for neuronal network activation. Ayahuasca administration increased perfusion in the right hemisphere anterior cingulate cortex (ACC) and medial frontal gyrus, bilaterally in the anterior insula and inferior frontal gyrus, and in the left amygdala and parahippocampal gyrus. These regions are thought to play key roles in interoception, body awareness, and emotional processing [ 199 , 200 ], well aligning with the acute subjective effects of ayahuasca [ 46 , 201 ]. A similar SPECT study in depressed patients showed significantly increased perfusion in the left nucleus accumbens (NAc), right insula and left subgenual area 8 h after ayahuasca treatment compared to baseline [ 29 ]. Additionally, acute reductions in depressive symptoms (80–180 min after administration) persisted up to three weeks. Previous neuroimaging studies (deep brain stimulation, PET, fMRI) have shown hypoactivity in precisely these regions in depressed patients, which rectified upon treatment with conventional antidepressants such as SSRIs or deep brain stimulation [ 202 , 203 , 204 , 205 , 206 ]. Post-acute results from the depressed group showed only partial overlap (in the right insula) with the acute effects of ayahuasca on cerebral perfusion in the healthy volunteer study [ 29 , 198 ], which might reflect changes in neuronal responsivity to the pharmacological challenge or time-dependent measurement differences.
In two task-based fMRI studies during acute DMT (i.v.), the first study showed no significant changes in blood oxygenation level dependent (BOLD) signal, despite the participants’ reduced reaction time to stimuli [ 207 ], whereas the second study showed signal reductions in brain regions associated with processing visual and auditory information in addition to reduced reaction time [ 208 ]. These combined behavioral and fMRI results recapitulated earlier behavioral findings with two different DMT doses [ 209 ]. Participants in another study with somewhat higher doses of i.v. DMT reported experiencing pronounced elementary and complex imagery [ 8 ], which might explain the reduced capability to focus on such attention-based tasks.
Another fMRI study investigating mental imagery during acute ayahuasca effects reported increased BOLD signal in many brain regions compared to baseline, including bilateral cuneus and left precuneus, lingual gyrus, fusiform, parahippocampal and temporal, occipital and frontal gyri [ 210 ]. These changes occurred during an imagery experience and may underly the often-reported vivid internal visual alterations with closed eyes. Partially overlapping results were reported in [ 198 ], and correspond to functional representations such as the peripheral visual field, retrieval of episodic memories, processing of contextual associations, and mental imagery. Changes in functional connectivity during mental imagery after ayahuasca intake, indicate alternations in the top down temporal information flow between frontal and occipital regions. Visions produced by ayahuasca seemingly arise in the primary visual cortex (V1) [ 210 ] and propagate to higher order visual regions. Another report of the same study sample showed changes in the default mode network (DMN) with task-based (verbal fluency) and with resting-state (rs) fMRI recordings [ 43 ]. Six of the nine pre-defined DMN regions showed significant activity decreases when comparing rest to task periods and ayahuasca to baseline. Two of the remaining DMN ROIs (left MFG and left MTG, involved in language processing [ 211 ]), also showed significant BOLD signal decreases. Additionally, functional connectivity declined within PCC/precuneus after ayahuasca intake. These findings suggest that experienced ayahuasca users achieve a brain state that occurs with decreased mind-wandering, allowing them to observe their thoughts and feelings without judgment, similar to experienced meditators [ 212 ].
In a follow-up analysis of the same rs-fMRI data increases of global entropy (Shannon entropy, expressing the uncertainty or variability in stochastic variables) were identified. Increases of local integration and decreases of global integration in various brain networks [ 213 ] imply that ayahuasca altered the modular structures of resting state networks. These results align with the entropic brain hypothesis , which proposes that psychedelic states entail higher entropy than ordinary waking consciousness [ 214 ].
A proton magnetic resonance spectroscopy ([ 1 H]-MRS) and rs-fMRI study with baseline and post-acute measurements one day after ayahuasca ingestion showed decreased glutamate + glutamine, creatinine + phosphocreatinine, and N -acetylaspartate + N -acetylaspartylglutamate signals in the PCC [ 215 ]. These lower metabolite levels indicate higher neuronal activity during acute ayahuasca intake [ 215 ]. Indeed, other psychedelics evoked decreased inhibitory alpha-waves in similar brain regions as in ayahuasca studies [ 141 , 216 , 217 ], and similar MRS changes occur in in patients successfully treated for depression with cognitive behavioral therapy (CBT) or SSRIs [ 218 ]. Complementary rs-fMRI measurements revealed enhanced crosstalk between the ACC (associated with executive and cognitive-emotional processing) and the PCC and limbic structures (highly relevant for emotion and memory processing), which may relate to the antidepressant effects of ayahuasca [ 215 ]. Parts of the salience (SAL; ACC) and the DMN (PCC) networks are habitually anti-correlated in normal waking consciousness [ 219 ], but enhanced coupling may occur during ayahuasca and other psychedelic experiences [ 220 ]. A later study replicated those findings of post-acute increased connectivity between the SAL and the DMN one day after administration of ayahuasca to healthy volunteers [ 221 ]. In accordance with previous reports, increased ACC connectivity within the salience network and decreased connectivity in the PCC within the DMN were identified [ 221 ]. A novel approach was adopted in a rs-fMRI study in a group setting with members of the Santo Daime church presenting “connectome fingerprints” for each participant, based on the idea that functional connectivity is more consistent within the same person across repeated scans than between different subjects [ 222 ]. Participants showed greater alignment between connectomes during the acute ayahuasca phase than during the placebo scans. After ayahuasca treatment, network stability decreased in the SAL, and increased in the dorsal attention network (DAN). Between-network stability mostly decreased from the SAL and visual network (VIS), extending to the other five large-scale brain networks defined by Yeo and colleagues [ 223 ].
Similarly, i.v. DMT administration decreased within-network integrity in five (VIS, somatomotor network (SM), DAN, fronto-parietal network (FP) and DMN) of the seven Yeo networks, and increased within-network functional connectivity in the SAL, FP and DMN [ 224 ]. An increased between-network functional connectivity between the FP, DMN, and SAL networks, and the other Yeo networks, suggest that DMT mainly affects networks integrating and processing higher cognitive functions. Additionally, DMT flattened the principal cortical gradient acutely [ 224 ] (which ranges from areas for processing sensory and motor information to regions subserving higher cognitive processing [ 225 ]). These findings suggest that DMT transiently dysregulates functional hierarchies, enabling greater cross-communication between brain regions and networks as compared to ordinary consciousness.
Another task-based fMRI study testing implicit aversive stimulation showed longer reaction times to aversive compared to neutral images at baseline, whereas during the ayahuasca scan reaction times did not differ according to emotional valence of the visual stimulus [ 226 ]. Furthermore, in the ayahuasca condition, there was decreased activation of the bilateral amygdala and increased activation of bilateral insula and right dorsolateral PFC upon exposure to aversive images. These findings align with previous ayahuasca studies, showing activation of regions involved in emotion processing, such as the amygdala, ACC, and insula, possibly related to the intensified emotional experience [ 29 , 198 ].
EEG studies with ayahuasca and DMT
The first open-label EEG study during the ayahuasca ritual among healthy members of the Santo Daime church reported increases in gamma power in the left posterior temporal cortex and the left occipital lobe with close eyes and increased gamma power in the central, parietal, and occipital lobes with open eyes compared to baseline [ 227 ]. Gamma is a high frequency (30–80 Hz) band modulated by external sensory inputs and internal processes such as working memory and attention; as such, their EEG findings bear some relation to fMRI findings of increased activity (e.g., in visual cortex, fusiform gyrus or prefrontal cortex) during a mental imagery task after ayahuasca administration [ 210 ]. A later study found a dose-dependent reduction in EEG power across all frequency bands, peaking between 90 and 120 min after ayahuasca administration [ 228 ]. Additional EEG analyses focussing on peak effects at 60 and 90 min after high doses [ 229 ] showed widespread bilateral decreases in alpha and delta power in somatosensory, auditory, and visual association cortices. At 90 min even greater reductions in beta, delta, and theta power occurred in cortical regions relevant for emotion and memory processing. Similar decreases in lower frequency (delta and theta) power have been observed after treatment with other psychedelics, or psychostimulants [ 230 , 231 ].
Delta waves usually increase during deep sleep, or in meditative and relaxed states, so decreased delta power might suggest an excitatory effect of ayahuasca [ 229 ]. This would be in accordance with animal studies showing excitatory postsynaptic potential and currents after psychedelics administration [ 232 ]. Although delta and theta power in EEG recordings was unchanged in a later study up to two hours after ayahuasca intake [ 137 ], alpha power declined in parieto-occipital regions at 50 min. Some cortical regions showed increased slow-gamma power between 75 and 125 min, while fast-gamma power had decreased in four clusters during the same time window. DMT and β -carboline plasma levels showed positive correlations with EEG power in the beta, gamma and delta bands, and a negative correlation with alpha power. Specifically, DMT and harmine levels correlated more strongly with early phase alpha power decreases, while the harmaline and THH concentrations correlated more strongly with the late phase gamma band increases. These changes in β -carboline profiles matched earlier pharmacokinetics findings [ 10 , 11 ].
In an EEG study using transfer entropy (TE) to measure directed information transfer ayahuasca administration resulted in decreased information flow from frontal to posterior brain regions and increased flow from posterior to frontal regions [ 233 ]. These changes, observed at various time points after administration, suggest that ayahuasca disrupts the usual neural hierarchies between higher order frontal regions and more sensory-related posterior regions, aligning with similar findings from fMRI studies with i.v. DMT [ 224 ].
An EEG study that tested the effects of ayahuasca and ketanserin in a 2 × 2 design found decreases in alpha, delta, and theta frequency bands after 90 min [ 141 ], much as in the above-mentioned earlier studies [ 137 , 228 , 229 ]. Ketanserin alone had opposite effects, with unchanged alpha power but increased delta and theta powers compared to placebo. When combined, ketanserin and ayahuasca led to stronger increases in delta and theta power, counteracting ayahuasca’s effects. Ketanserin before ayahuasca reduced, but did not completely block all subjective effects, possibly related to changes in EEG bands.
Performance of a cognitive mismatch negativity (MMN, a brain response to violations of a rule) EEG task decreased dose-dependently during i.v. DMT administrations compared to baseline [ 234 ]. After the low DMT dose, there was diminished N1 peak amplitude (~ 150 ms after stimulus), indicating decreased attention to visual stimuli [ 235 ], and attenuated MMN signal in the right hemisphere. Similarly, psilocybin treatment also reduced N1 peak activity, albeit with stronger effects on MMN [ 236 , 237 ].
A more recent EEG study with i.v. DMT showed significantly increased signal diversity and delta and gamma powers, while decreasing alpha and posterior beta powers, correlating with intensity ratings and DMT plasma levels [ 238 ]. A cortical travelling wave analysis revealed increased forward waves (FW) and decreased backward waves (BW) [ 239 ]. After DMT administration, the frequency of the travelling waves decreased for alpha and beta and increased for delta and theta, matching previously reported frequency band power changes. These changes resembled those seen during visual stimulation [ 240 ], suggesting a mechanism for DMT-induced visual hallucinations [ 8 , 241 ]. Another analysis modelled the relationship between alpha and beta power, signal complexity and simulated DMT plasma levels, identifying specific concentrations evoking half-maximal (IC 50 ) band reductions in alpha (71 nM) and beta power (137 nM), and the EC 50 for signal complexity (54 nM). These results constitute the first dose–response relationship for EEG signal strength with DMT.
EEG recordings after self-administration of DMT by smoking in a naturalistic setting recapitulated findings [ 238 ] of a widespread reduction in alpha power lasting several minutes, along with reduced delta and gamma power in occipital, parietal, temporal and antero-central regions during the same time window [ 242 ]. In a separate reanalysis of these data, the same pattern of power changes was found alongside a negative correlation with subjective effects only with theta power changes [ 243 ].
An additional analysis of the study presented above [ 224 ] focused on the relationships between EEG and simultaneous fMRI findings after i.v. DMT administration. EEG data showed reduced alpha and beta power, decreased fractal spectral power below 30 Hz, and increased signal complexity. Significant negative correlations were found between intensity ratings and plasma DMT concentrations with alpha- and beta-power and positive correlations with delta- and theta-power, much as reported in [ 238 ]. The cross-model analysis showed positive correlations between frontal delta power and negative correlations between parietal alpha-power with GFC in most RSNs, along with positive correlations between gamma power and signal diversity in a few RSNs. This study reinforced preceding EEG findings with additional fMRI data, demonstrating the benefits of multimodal neuroimaging.
In reviewing EEG studies on ayahuasca and DMT, two main findings consistently appear: (1) a reduction in alpha frequency power, and (2) an increase in signal complexity. These effects occur 60–120 min after ayahuasca administration and shortly after i.v. or inhaled DMT [ 137 , 141 , 224 , 228 , 229 , 238 , 242 , 243 ]. Reduced alpha power has been linked to increased brain metabolism [ 244 , 245 , 246 ], which could explain the increased BOLD signal in the visual cortex [ 210 ], and the vivid visual effects often reported with these substances. The alpha band is the most prominent feature of resting-state EEG recordings in adults [ 247 ], and is linked to high-level psychological functioning [ 248 , 249 ] and top-down brain regulation [ 250 , 251 ], both of which are generally modified by psychedelics [ 224 , 233 , 252 , 253 ]. While changes in other frequency bands are less consistent, the increase in signal complexity supports the idea of decreased top-down regulation and hierarchical processing of neural information during the psychedelic state [ 214 , 252 ].
Neuroimaging and EEG studies on DMT and ayahuasca vary widely in their designs, dosages, administration routes, and settings, which can make comparisons difficult. However, many studies focused on resting-state EEG or fMRI, which are useful for examining brain activity without specific tasks and can be easily compared. Despite these caveats, these studies have informed several models of psychedelic action emerging over the past few years. These models include the entropic brain hypotheses as noted above [ 214 ] and its generalization, the REBUS (relaxed beliefs under psychedelics and the anarchic brain) model [ 252 ], the cortico-striato-thalamocortical (CSTC) model [ 254 ], the strong priors (SP) model [ 255 ], and the cortico-claustro-cortical (CCC) model [ 256 ]. The CSTC and the CCC model mainly rely on the assumption that 5-HT 2A receptor agonism is the key driver for psychedelic effects, which is less than certain for the case of DMT/harmine. The REBUS model proposes that psychedelics reduce the influence of top-down processes (like expectations or prior beliefs) and thereby enhance bottom-up sensory and emotional information flow potentially facilitating therapeutic change processes. We prefer the REBUS model because it explains several observed effects such as the reduction of a cortical gradient (fMRI), modulations of RSNs (especially the DMN), increased signal complexity (EEG) or altered direction of cortical traveling waves in the alpha band [ 257 ].
For a comprehensive integration of these models into general neuroscientific research on psychedelics, see [ 258 ]. Several systematic reviews have examined neuroimaging studies with various psychedelic substances, e.g., Gattuso et al. [ 259 ] and McCulloch et al. [ 260 ]. These reviews highlight the modulation of the DMN and resting-state brain activity under the influence of psychedelics, using various imaging techniques (e.g. fMRI, EGG, MEG). However, there is a notable lack of molecular imaging studies specifically examining the actions of DMT and ayahuasca.
Long-term psychological and neuroanatomical changes after ayahuasca use
In a cross-sectional study, Bouso et al. [ 261 ] compared long-term ayahuasca users (n = 127) of various ayahuasca churches in Brazil with a religious control group (n = 115) that abstains from substance consumption. Psychometric assessments were conducted at study inclusion (T1) and one year later (T2). The ayahuasca group showed significantly lower Harm Avoidance and Self-Directedness, higher Self-Transcendence, and lower general subjective symptoms, which suggests that consumption in religious contexts is beneficial for mental and physical health. Additionally, ayahuasca users scored higher on all subscales of the Spiritual Orientation Inventory [ 262 ] at both time-points, indicating a reinforcement of spiritual beliefs and attitudes. They also performed better in neuropsychological tests of conflict monitoring, executive function, and working memory at both time-points.
Structural MRI provides quantitative measures of brain volume and cortical thickness, which can indicate structural changes due to aging, neurotoxicity, or neuroplasticity. Bouso et al. [ 263 ] reported on cortical thickness in experienced ayahuasca users from the Santo Daime with a matched control group. The habitual ayahuasca users showed significant cortical thinning in middle and inferior frontal gyrus, precuneus, superior frontal gyrus, PCC, and superior occipital gyrus, along with cortical thickening in the precentral gyrus and ACC. Cortical thinning in the PCC negatively correlated with lifetime and years of ayahuasca use. Neuropsychological tests revealed the same differences as the cross-sectional study mentioned above. The cortical thinning in key nodes of the DMN might imply long-term downregulation of DMN activity, although no direct evidence links this to reduced within-DMN connectivity [ 43 , 224 ]. The negative correlation of PCC thickness and self-transcendence might imply an anatomical basis for the increased religiosity often reported by ayahuasca users. Overall, Bouso’s studies suggest that regular, long-term ayahuasca use in religious contexts benefits brain health, though these findings may not necessarily apply to a secular or psychiatric use. The general abstinence from other drugs among ayahuasca church members may support the cognitive benefits attributed to ritual use.
Another analysis of the same MRI study tested the hypothesis that regular ayahuasca use would lead to thickening of fiber tracts in the corpus callosum, the bundle connecting the cerebral hemispheres [ 264 ]. Results showed thickening in the isthmus of the corpus callosum, correlating with ayahuasca use frequency, although these findings did not hold after multiple testing corrections. The implicated callosal regions are positioned to mediate increased interhemispheric connectivity between motor and somatosensory regions [ 265 ]. Obtaining such an effect might be beneficial for motor function, various neurodegenerative disorders, e.g. amyotrophic lateral sclerosis (ALS), and for stroke rehabilitation [ 265 , 266 ].
A more recent cross-sectional structural MRI study assessed morphometric similarity (MS), an approach whereby anatomical connectivity is analyzed by considering multiple brain structural features (e.g. grey matter volume, and cortical curvature and thickness) [ 267 ], in 24 frequent ayahuasca users from the Santo Daime church and 24 matched controls [ 268 ]. The ayahuasca group showed overall reduced MS compared to controls, with lower MS in sensorimotor cortices (inferior frontal gyrus, precuneus, pre and post central gyrus) and higher MS in the orbitofrontal, entorhinal, cingulate, and anterior insular cortices. Lower MS indicates decreased anatomical connectivity between a region and the rest of the cortex [ 268 ]. The findings of increased MS in the ACC align with an earlier MRI study showing thinning [ 263 ]. MS analysis with the seven RSNs defined by Yeo and colleagues [ 223 ] revealed reduced MS in the sensorimotor, dorsal attention, and default mode networks for the ayahuasca group, with increased MS in the limbic network. Additional correlations with gene expression maps in the ayahuasca group revealed 18 genes relevant to DMT or ayahuasca effects (with either positive or negative weightings) on MS findings. Notably, 5-HT 2A receptor gene downregulation in sensorimotor cortices correlated with lower MS, suggesting that repeated ayahuasca use may lead to sustained downregulation and desensitization, similar to LSD tolerance [ 269 ]. Since ayahuasca or DMT do not seem to evoke rapid tolerance [ 62 ], it is questionable if such 5-HT 2A receptor downregulation could mediate tolerance. Earlier neuropsychology studies showed that regular ayahuasca users had better integrity of executive functions than less experienced users [ 270 ], which might relate to 5-HT 2A receptor desensitization or downregulation, a matter for future PET studies.
Pharmacological models alone may not fully explain the anatomic/functional associations observed in ayahuasca studies. For example, religiosity in Christian church members showed associations with a widespread pattern of greater cortical thickness [ 271 ], compared to the more diverse findings of cortical thickness changes in ayahuasca users. The cross-sectional studies of Santo Daime church members, who abstain from other drugs, provide a unique opportunity to study repeated ayahuasca use. However, these cross-sectional studies cannot infer causality. Larger, longitudinal studies are needed to confirm the structural and functional brain changes linked to ayahuasca use.
Conclusions and future directions
With this narrative review and synthesis, we summarize the current state of research with DMT, β -carbolines, and ayahuasca. While DMT is the main psychedelic constituent, the diverse β -carboline alkaloids in ayahuasca contribute to its unique (adverse) effects, creating an entourage effect that distinguishes it from synthetic formulations. The combination of DMT with MAO inhibitors enhances its bioavailability and duration and offers alternatives to inhaled or injectable routes of administration, which lead to short but intense DMT experiences. We emphasize the need to distinguish between a reductionist view of ayahuasca as a mixture of chemical substances and its full context as a cultural practice, often affiliated with traditional and syncretic religions. The findings presented herein mostly fall within the broad field of neurobiology, and do not adequately accommodate the cultural and botanical knowledge associated with indigenous usage of ayahuasca for therapeutic and ritualistic purposes.
Current understanding does not definitively implicate a singular molecular target that could explain the subjective effects of DMT or ayahuasca. Key candidates include several serotonin receptor subtypes (5-HT 1A/2A/2C and potentially others), an indirect influence on dopamine transmission due to β- carbolines, and potential roles of TrkB and sigma-1 receptors. Better qualifying the importance of 5-HT 2A receptor activation by DMT (alone or in combination with MAOIs) shall call for dedicated pre-clinical and clinical molecular imaging studies.
Neuroimaging and EEG studies have consistently shown reductions in alpha frequency power and increased signal complexity after ayahuasca or DMT administration. These findings are in line with brain models of decreased top–down regulation and enhanced neural communication during the psychedelic state. Functional neuroimaging studies have revealed changes in brain network dynamics, such as increased connectivity between the salience and default mode networks, and activations of brain areas associated with processing of emotions and autobiographical memories. Understanding these mechanisms is crucial for developing targeted therapeutic applications. The preponderance of imaging studies and clinical studies have been exploratory with open-label designs. There is a pressing need for larger-scale, controlled clinical trials to establish the dose-dependence and persistence of long-term therapeutic benefits and neurobiological effects induced by ayahuasca or DMT. Such studies should integrate clinical scoring with advanced imaging methods. We also see a need for comparative studies with other classical psychedelics, aiming to understand better the neurobiological basis of their differing phenomenology. Promising findings that DMT and ayahuasca evoke neuroplastic effects call for consolidation with comparable results seen with other psychedelic compounds. The bulk of such research has hitherto entailed studies in vitro and in preclinical research, without translation to the human clinical context. Multimodal neuroimaging techniques such as diffusion tensor imaging (DTI) could serve to investigate changes in white matter tracts in conjunction with MRS to follow changes in specific neurometabolites acutely or over time [ 272 ]. Recent advances in neuroimaging data processing, e.g. the development of a modality-overarching neuroimaging data structure [ 273 ], standardization of fMRI preprocessing pipelines [ 274 ], and the development of sophisticated computational approaches should enable streamlining and standardization of neuroimaging results to facilitate easier interpretation between studies.
Additionally, molecular imaging studies, including PET, are essential to further explore receptor occupancy and neuroplasticity mechanisms in vivo. Neuroplasticity in response to ayahuasca or its constituents might be amenable to investigation by PET with a ligand for synaptic vesicle protein 2A (SV2A), as shown autoradiographically for the case of psilocybin in experimental animals [ 275 ]. PET studies with the serotonin 5-HT 2A/2C receptor agonist radioligand [ 11 C]Cimbi-36 [ 276 ] (i.e., the psychedelic phenylethylamine generally known as 25B-NBOMe [ 277 ]) may be better suited than antagonist radioligands for establishing competition from the exogenous agonist DMT in vivo, as is the case for dopamine D 2/3 receptors [ 278 ]. Similarly, an agonist PET ligand for 5-HT 1A receptors might serve to detect occupancy by DMT at this receptor in human brain. As noted above, the complex alkaloid composition of ayahuasca calls for consideration of the entourage effect, a concept originally known from cannabis research [ 67 ], and indeed in consideration of the polypharmacology of tobacco smoke, which evokes inhibition of MAO-A in human organs to [ 11 C]clorgyline PET [ 279 ]. There were no effects in a pilot study of low dose harmine/DMT on energy metabolism in rat brain to [ 18 F]FDG PET [ 103 ], but we are currently undertaking human [ 18 F]FDG PET studies of pharmahuasca. Results of this ongoing neuroenergetics study could prove informative regarding the interpretation of the extensive fMRI/EEG literature on ayahuasca reviewed above.
In conclusion, the therapeutic potential of ayahuasca constituents to promote neuroplasticity and treat neuropsychiatric disorders, including depression, addiction, and PTSD, is gaining empirical support. Additionally, early evidence suggests a potential role of DMT in the treatment of acute brain injury such as ischemic stroke, which opens promising paths for future pharmacotherapeutic developments. Standardized formulations of DMT and harmala alkaloids present certain advantages for clinical investigations and basic research into the molecular pathways and mechanisms of action by offering more predictable pharmacokinetic and pharmacodynamic profiles, as well as better control of potential adverse effects, compared with botanical ayahuasca preparations. In addition to the molecular and neurophysiological perspective, we note the importance of processes of psychological change, alongside clinical and contextual factors such as supportive set and setting. While extending beyond this review’s original scope, we hold that such considerations are essential for obtaining a comprehensive understanding of the therapeutic potential of ayahuasca.
Data availability
Not applicable.
DMT is also present in a hallucinogenic snuff (known as yopo) derived from seeds of the South American trees Anadenanthera peregrina or A. columbina , along with the psychoactive tryptamines 5-methoxy-DMT (5-MeO-DMT) and 5-hydroxy-DMT (bufotenin) [ 280 ].
Nichols DE (2016) Psychedelics. Pharmacol Rev 68:264–355. https://doi.org/10.1124/pr.115.011478
Article PubMed PubMed Central CAS Google Scholar
Shulgin AT, Shulgin A (1991) PIHKAL, a chemical love story. Transform Press, Berkeley
Google Scholar
Shulgin AT, Shulgin A (1997) TIHKAL, the continuation. Transform Press
Carod-Artal FJ (2015) Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurología (English Edition) 30:42–49. https://doi.org/10.1016/J.NRLENG.2011.07.010
Article CAS Google Scholar
Riba J, Rodríguez-Fornells A, Urbano G et al (2001) Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology 154:85–95. https://doi.org/10.1007/s002130000606
Article PubMed CAS Google Scholar
Riba J, McIlhenny EH, Bouso JC, Barker SA (2015) Metabolism and urinary disposition of N , N -dimethyltryptamine after oral and smoked administration: a comparative study. Drug Test Anal 7:401–406. https://doi.org/10.1002/dta.1685
Vogt SB, Ley L, Erne L et al (2023) Acute effects of intravenous DMT in a randomized placebo-controlled study in healthy participants. Transl Psychiatry 13:172. https://doi.org/10.1038/s41398-023-02477-4
Luan LX, Eckernäs E, Ashton M et al (2023) Psychological and physiological effects of extended DMT. J Psychopharmacol. https://doi.org/10.1177/02698811231196877
Article PubMed PubMed Central Google Scholar
Hofmann A (1979) How LSD originated. J Psychedelic Drugs 11:53–60. https://doi.org/10.1080/02791072.1979.10472092
Callaway JC, McKenna DJ, Grob CS et al (1999) Pharmacokinetics of Hoasca alkaloids in healthy humans. J Ethnopharmacol 65:243–256. https://doi.org/10.1016/s0378-8741(98)00168-8
Grob CS, McKenna DJ, Callaway JC et al (1996) Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nervous Mental Dis 184:86–94. https://doi.org/10.1097/00005053-199602000-00004
Barbosa PCR, Giglio JS, Dalgalarrondo P (2005) Altered states of consciousness and short-term psychological after-effects induced by the first time ritual use of ayahuasca in an urban context in Brazil. J Psychoactive Drugs 37:193–201. https://doi.org/10.1080/02791072.2005.10399801
Article PubMed Google Scholar
Luna LE, Amaringo P (1999) Ayahuasca visions: the religious iconography of a Peruvian Shaman. North Atlantic Books, Berkely
Franquesa A, Sainz-Cort A, Gandy S et al (2018) Psychological variables implied in the therapeutic effect of ayahuasca: a contextual approach. Psychiatry Res 264:334–339. https://doi.org/10.1016/j.psychres.2018.04.012
Shanon B (2014) Moments of insight, healing, and transformation: a cognitive phenomenological analysis. In: Labate BC, Cavnar C (eds) The therapeutic use of ayahuasca. Springer, Berlin, pp 59–75
Chapter Google Scholar
Shanon B (2003) Altered states and the study of consciousness—the case of ayahuasca. J Mind Behav 24:125–153
Kavenská V, Simonová H (2015) Ayahuasca tourism: participants in shamanic rituals and their personality styles, motivation, benefits and risks. J Psychoactive Drugs 47:351–359. https://doi.org/10.1080/02791072.2015.1094590
Bresnick T, Levin R (2006) Phenomenal qualities of ayahuasca ingestion and its relation to fringe consciousness and personality. J Conscious Stud 13:5–24
Kjellgren A, Eriksson A, Norlander T (2009) Experiences of encounters with ayahuasca—“the vine of the soul.” J Psychoactive Drugs 41:309–315. https://doi.org/10.1080/02791072.2009.10399767
Politi M, Tresca G, Menghini L, Ferrante C (2022) Beyond the psychoactive effects of ayahuasca: cultural and pharmacological relevance of its emetic and purging properties. Planta Med 88:1275–1286. https://doi.org/10.1055/a-1675-3840
Labate BC, Cavnar C (2014) Ayahuasca shamanism in the Amazon and beyond. Oxford University Press, New York
Book Google Scholar
Anderson BT (2012) Ayahuasca as antidepressant? Psychedelics and styles of reasoning in psychiatry. Anthropol Conscious 23:44–59. https://doi.org/10.1111/j.1556-3537.2012.01056.x
Article Google Scholar
Barbosa PC, Cazorla IM, Giglio JS, Strassman R (2009) A six-month prospective evaluation of personality traits, psychiatric symptoms and quality of life in ayahuasca-naive subjects. J Psychoactive Drugs 41:205–212. https://doi.org/10.1080/02791072.2009.10400530
Fortunato JJ, Réus GZ, Kirsch TR et al (2010) Effects of β-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res Bull 81:491–496. https://doi.org/10.1016/j.brainresbull.2009.09.008
Fortunato JJ, Réus GZ, Kirsch TR et al (2010) Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus. J Neural Transm 117:1131–1137. https://doi.org/10.1007/s00702-010-0451-2
Ruffell SGD, Netzband N, Tsang W et al (2021) Ceremonial ayahuasca in amazonian retreats—mental health and epigenetic outcomes from a six-month naturalistic study. Front Psychiatry. https://doi.org/10.3389/fpsyt.2021.687615
Sarris J, Perkins D, Cribb L et al (2021) Ayahuasca use and reported effects on depression and anxiety symptoms: an international cross-sectional study of 11,912 consumers. J Affect Disord Rep 4:100098. https://doi.org/10.1016/j.jadr.2021.100098
de Osório FL, Sanches RF, Macedo LR et al (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry 37:13–20. https://doi.org/10.1590/1516-4446-2014-1496
Sanches RF, de Lima OF, Dos Santos RG et al (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol 36:77–81. https://doi.org/10.1097/JCP.0000000000000436
Palhano-Fontes F, Barreto D, Onias H et al (2019) Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med 49:655–663. https://doi.org/10.1017/S0033291718001356
Domínguez-Clavé E, Soler J, Elices M et al (2022) Ayahuasca may help to improve self-compassion and self-criticism capacities. Human Psychopharmacol. https://doi.org/10.1002/hup.2807
van Oorsouw K, Toennes SW, Ramaekers JG (2022) Therapeutic effect of an ayahuasca analogue in clinically depressed patients: a longitudinal observational study. Psychopharmacology 239:1839–1852. https://doi.org/10.1007/s00213-021-06046-9
Zeifman RJ, Palhano-Fontes F, Hallak J et al (2019) The impact of ayahuasca on suicidality: results from a randomized controlled trial. Front Pharmacol. https://doi.org/10.3389/fphar.2019.01325
Thomas G, Lucas P, Capler N et al (2013) Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev 6:30–42. https://doi.org/10.2174/15733998113099990003
O’Shaughnessy DM, Berlowitz I, Rodd R et al (2021) Within-treatment changes in a novel addiction treatment program using traditional Amazonian medicine. Ther Adv Psychopharmacol 11:204512532098663. https://doi.org/10.1177/2045125320986634
Berlowitz I, Walt H, Ghasarian C et al (2019) Short-term treatment effects of a substance use disorder therapy involving traditional Amazonian medicine. J Psychoactive Drugs 51:323–334. https://doi.org/10.1080/02791072.2019.1607956
Barbosa PCR, Tófoli LF, Bogenschutz MP et al (2018) Assessment of alcohol and tobacco use disorders among religious users of ayahuasca. Front Psychiatry. https://doi.org/10.3389/fpsyt.2018.00136
Loizaga-Velder A, Verres R (2014) Therapeutic effects of ritual ayahuasca use in the treatment of substance dependence—qualitative results. J Psychoactive Drugs 46:63–72. https://doi.org/10.1080/02791072.2013.873157
Renelli M, Fletcher J, Tupper KW et al (2020) An exploratory study of experiences with conventional eating disorder treatment and ceremonial ayahuasca for the healing of eating disorders. Eat Weight Disord 25:437–444. https://doi.org/10.1007/s40519-018-0619-6
Lafrance A, Loizaga-Velder A, Fletcher J et al (2017) Nourishing the spirit: exploratory research on ayahuasca experiences along the continuum of recovery from eating disorders. J Psychoactive Drugs 49:427–435. https://doi.org/10.1080/02791072.2017.1361559
González D, Carvalho M, Cantillo J et al (2019) Potential use of ayahuasca in grief therapy. Omega 79:260–285. https://doi.org/10.1177/0030222817710879
González D, Cantillo J, Pérez I et al (2020) Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacology 237:1171–1182. https://doi.org/10.1007/s00213-019-05446-2
Palhano-Fontes F, Andrade KC, Tofoli LF et al (2015) The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One 10:e0118143. https://doi.org/10.1371/journal.pone.0118143
Dakic V, de Maciel RM, Drummond H, et al (2016) Harmine stimulates proliferation of human neural progenitors. PeerJ 4:e2727. https://doi.org/10.7717/peerj.2727
Katchborian-Neto A, Santos WT, Nicácio KJ et al (2020) Neuroprotective potential of ayahuasca and untargeted metabolomics analyses: applicability to Parkinson’s disease. J Ethnopharmacol 255:112743. https://doi.org/10.1016/j.jep.2020.112743
Maia LO, Daldegan-Bueno D, Wießner I et al (2023) Ayahuasca’s therapeutic potential: What we know – and what not. Eur Neuropsychopharmacol 66:45–61. https://doi.org/10.1016/j.euroneuro.2022.10.008
Dos Santos RG, Osorio FL, Crippa JA, Hallak JE (2016) Antidepressive and anxiolytic effects of ayahuasca: a systematic literature review of animal and human studies. Braz J Psychiatry 38:65–72. https://doi.org/10.1590/1516-4446-2015-1701
Perkins D, Schubert V, Simonová H et al (2021) Influence of context and setting on the mental health and wellbeing outcomes of ayahuasca drinkers: results of a large international survey. Front Pharmacol. https://doi.org/10.3389/fphar.2021.623979
Gable RS (2007) Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 102:24–34. https://doi.org/10.1111/j.1360-0443.2006.01652.x
Rodríguez L, López A, Moyna G et al (2022) New insights into the chemical composition of ayahuasca. ACS Omega 7:12307–12317. https://doi.org/10.1021/acsomega.2c00795
Estrella-Parra EA, Almanza-Pérez JC, Alarcón-Aguilar FJ (2019) Ayahuasca: uses, phytochemical and biological activities. Nat Prod Bioprospect 9:251–265. https://doi.org/10.1007/s13659-019-0210-5
McKenna DJ, Towers GH, Abbott F (1984) Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol 10:195–223. https://doi.org/10.1016/0378-8741(84)90003-5
Kaasik H, Souza RCZ, Zandonadi FS et al (2021) Chemical composition of traditional and analog ayahuasca. J Psychoactive Drugs 53:65–75. https://doi.org/10.1080/02791072.2020.1815911
Politi M, Friso F, Saucedo G, Torres J (2021) Traditional Use of Banisteriopsis caapi alone and its application in a context of drug addiction therapy. J Psychoactive Drugs 53:76–84. https://doi.org/10.1080/02791072.2020.1820641
Rodd R (2008) Reassessing the cultural and psychopharmacological significance of Banisteriopsis caapi : preparation, classification and use among the Piaroa of Southern Venezuela. J Psychoactive Drugs 40:301–307. https://doi.org/10.1080/02791072.2008.10400645
Ott J (1999) Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs 31:171–177. https://doi.org/10.1080/02791072.1999.10471741
Berlowitz I, O’Shaughnessy DM, Heinrich M et al (2022) Teacher plants—Indigenous Peruvian-Amazonian dietary practices as a method for using psychoactives. J Ethnopharmacol 286:114910. https://doi.org/10.1016/J.JEP.2021.114910
Wang Y-H, Samoylenko V, Tekwani BL et al (2010) Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson’s disease. J Ethnopharmacol 128:662–671. https://doi.org/10.1016/j.jep.2010.02.013
Freedland CS, Mansbach RS (1999) Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol Depend 54:183–194. https://doi.org/10.1016/s0376-8716(98)00154-9
Barker SA (2018) N , N -dimethyltryptamine (DMT), an endogenous hallucinogen: past, present, and future research to determine its role and function. Front Neurosci 12:536. https://doi.org/10.3389/fnins.2018.00536
Riba J, Valle M, Urbano G et al (2003) Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther 306:73–83. https://doi.org/10.1124/jpet.103.049882
Dos Santos RG, Grasa E, Valle M et al (2012) Pharmacology of ayahuasca administered in two repeated doses. Psychopharmacology 219:1039–1053. https://doi.org/10.1007/s00213-011-2434-x
Palhano-Fontes F, Alchieri JC, Oliveira JPM et al (2014) The therapeutic potentials of ayahuasca in the treatment of depression. In: Labate BC, Cavnar C (eds) The therapeutic use of ayahuasca. Springer, Berlin, pp 23–39
Buckholtz NS, Boggan WO (1977) Inhibition by β-carbolines of monoamine uptake into a synaptosomal preparation: structure-activity relationships. Life Sci 20:2093–2100. https://doi.org/10.1016/0024-3205(77)90190-4
Herraiz T, Chaparro C (2005) Human monoamine oxidase is inhibited by tobacco smoke: β-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun 326:378–386. https://doi.org/10.1016/j.bbrc.2004.11.033
Chaurasiya ND, Leon F, Muhammad I, Tekwani BL (2022) Natural products inhibitors of monoamine oxidases-potential new drug leads for neuroprotection, neurological disorders, and neuroblastoma. Molecules. https://doi.org/10.3390/molecules27134297
Ben-Shabat S, Fride E, Sheskin T et al (1998) An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 353:23–31. https://doi.org/10.1016/S0014-2999(98)00392-6
Dominguez-Clave E, Soler J, Elices M et al (2016) Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull 126:89–101. https://doi.org/10.1016/j.brainresbull.2016.03.002
McKenna DJ (2004) Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther 102:111–129. https://doi.org/10.1016/j.pharmathera.2004.03.002
Santos BWL, de Oliveira RC, Sonsin-Oliveira J et al (2020) Biodiversity of β-carboline profile of Banisteriopsis caapi and ayahuasca, a plant and a brew with Neuropharmacological potential. Plants 9:870. https://doi.org/10.3390/plants9070870
de Oliveira Silveira G, Guimarães Dos Santos R, Rebello Lourenço F et al (2020) Stability evaluation of DMT and harmala alkaloids in ayahuasca tea samples. Molecules. https://doi.org/10.3390/molecules25092072
Rivier L, Lindgren J-E (1972) “Ayahuasca”, the South American hallucinogenic drink: an ethnobotanical and chemical investigation. Econ Bot 26:101–129. https://doi.org/10.1007/BF02860772
Callaway JC (2005) Various alkaloid profiles in decoctions of Banisteriopsis caapi . J Psychoactive Drugs 37:151–155. https://doi.org/10.1080/02791072.2005.10399796
Pires APS, De Oliveira CDR, Moura S et al (2009) Gas chromatographic analysis of dimethyltryptamine and β -carboline alkaloids in ayahuasca, an amazonian psychoactive plant beverage. Phytochem Anal 20:149–153. https://doi.org/10.1002/pca.1110
Souza RCZ, Zandonadi FS, Freitas DP et al (2019) Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J Chromatogr B 1124:197–203. https://doi.org/10.1016/j.jchromb.2019.06.014
Lanaro R, CalemiTogni DBALR et al (2015) Ritualistic use of ayahuasca versus street use of similar substances seized by the police: a key factor involved in the potential for intoxications and overdose? J Psychoactive Drugs 47:132–139. https://doi.org/10.1080/02791072.2015.1013202
Ott J (1993) Pharmacotheon: entheogenic drugs, their plant sources and history. Natural Products Co., Kennewick
Huang Q, Li L, Zheng M et al (2018) The tryptophan decarboxylase 1 gene from Aegilops variabilis no. 1 regulate the resistance against cereal cyst nematode by altering the downstream secondary metabolite contents rather than auxin synthesis. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01297
Gill RIS, Ellis BE, Isman MB (2003) Tryptamine-induced resistance in tryptophan decarboxylase transgenic poplar and tobacco plants against their specific herbivores. J Chem Ecol 29:779–793. https://doi.org/10.1023/A:1022983529555
Clark M (2019) Soma and Haoma: Ayahuasca analogues from the late bronze age. J Psychedelic Stud 3:104–116. https://doi.org/10.1556/2054.2019.013
Moloudizargari M, Mikaili P, Aghajanshakeri S et al (2013) Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 7:199. https://doi.org/10.4103/0973-7847.120524
Mina CN, Farzaei MH, Gholamreza A (2015) Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med 35:104–109. https://doi.org/10.1016/s0254-6272(15)30016-9
Savoldi R, Roazzi A, de Oliveira Sales RC (2023) Mystical and ego-dissolution experiences in Ayahuasca and Jurema holistic rituals: an exploratory study. Int J Psychol Relig 33:332–360. https://doi.org/10.1080/10508619.2023.2185369
de Souza RSO, de Albuquerque UP, Monteiro JM, de Amorim ELC (2008) Jurema-Preta ( Mimosa tenuiflora [Willd.] Poir.): a review of its traditional use, phytochemistry and pharmacology. Braz Arch Biol Technol 51:937–947. https://doi.org/10.1590/S1516-89132008000500010
Naranjo C (1974) The healing journey: new approaches to consciousness, 1st edn. Pantheon Books, New York
Ott J (1994) Ayahuasca Analogues: Pangæan Entheogens. Natural Products Company
St John G (2016) Aussiewaska: a cultural history of changa and ayahuasca analogues in Australia. In: Labate BC, Cavnar C, Gearin AK (eds) The world ayahuasca diaspora. Taylor & Francis Group, Routledge, pp 143–162
Coe MA, Gaoue OG (2023) Increased clonal growth in heavily harvested ecosystems failed to rescue ayahuasca lianas from decline in the Peruvian Amazon rainforest. J Appl Ecol 60:2105–2117. https://doi.org/10.1111/1365-2664.14488
Dornbierer DA, Marten L, Mueller J et al (2023) Overcoming the clinical challenges of traditional ayahuasca: a first-in-human trial exploring novel routes of administration of N,N-dimethyltryptamine and harmine. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1246892
Axelrod J (1961) Enzymatic formation of psychotomimetic metabolites from normally occurring compounds. Science 134:343–343. https://doi.org/10.1126/science.134.3475.343
Thompson MA, Moon E, Kim UJ et al (1999) Human indolethylamine N-methyltransferase: cDNA cloning and expression, gene cloning, and chromosomal localization. Genomics 61:285–297. https://doi.org/10.1006/geno.1999.5960
Dean JG, Liu T, Huff S et al (2019) Biosynthesis and extracellular concentrations of N,N-dimethyltryptamine (DMT) in mammalian brain. Sci Rep 9:9333. https://doi.org/10.1038/s41598-019-45812-w
Glynos NG, Carter L, Lee SJ et al (2023) Indolethylamine N-methyltransferase (INMT) is not essential for endogenous tryptamine-dependent methylation activity in rats. Sci Rep 13:280. https://doi.org/10.1038/s41598-023-27538-y
Fitzgerald PJ (2009) Neuromodulating mice and men: Are there functional species differences in neurotransmitter concentration? Neurosci Biobehav Rev 33:1037–1041. https://doi.org/10.1016/j.neubiorev.2009.04.003
Egger K, Gudmundsen F, Jessen NS et al (2023) A pilot study of cerebral metabolism and serotonin 5-HT2A receptor occupancy in rats treated with the psychedelic tryptamine DMT in conjunction with the MAO inhibitor harmine. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1140656
Körmöczi T, Szabó Í, Farkas E et al (2020) Heart-cutting two-dimensional liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry for determination of N,N-dimethyltryptamine in rat plasma and brain; Method development and application. J Pharm Biomed Anal 191:113615. https://doi.org/10.1016/j.jpba.2020.113615
Barker SA (2022) Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT. Psychopharmacology 239:1749–1763. https://doi.org/10.1007/s00213-022-06065-0
Cameron LP, Olson DE (2018) Dark classics in chemical neuroscience: N,N-dimethyltryptamine (DMT). ACS Chem Neurosci 9:2344–2357. https://doi.org/10.1021/acschemneuro.8b00101
Cohen I, Vogel WH (1972) Determination and physiological disposition of dimethyltryptamine and diethyltryptamine in rat brain, liver and plasma. Biochem Pharmacol 21:1214–1216. https://doi.org/10.1016/0006-2952(72)90119-0
Sitaram BR, Lockett L, Talomsin R et al (1987) In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem Pharmacol 36:1509–1512. https://doi.org/10.1016/0006-2952(87)90118-3
Yanai K, Ido T, Ishiwata K et al (1986) In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N,N-[11C]dimethyltryptamine. Eur J Nucl Med 12:141–146. https://doi.org/10.1007/BF00276707
Vitale AA, Pomilio AB, Cañellas CO et al (2011) In vivo long-term kinetics of radiolabeled N,N-dimethyltryptamine and tryptamine. J Nucl Med 52:970–977. https://doi.org/10.2967/JNUMED.110.083246
Egger K, Gudmundsen F, Jessen NS et al (2023) A pilot study of cerebral metabolism and serotonin 5-HT2A receptor occupancy in rats treated with the psychedelic tryptamine DMT in conjunction with the MAO inhibitor harmine. Front Pharmacol 14:1140656. https://doi.org/10.3389/fphar.2023.1140656
Frecska E, Szabo A, Winkelman MJ et al (2013) A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J Neural Transm (Vienna) 120:1295–1303. https://doi.org/10.1007/s00702-013-1024-y
Teleanu RI, Niculescu A-G, Roza E et al (2022) Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int J Mol Sci. https://doi.org/10.3390/ijms23115954
Jiménez JH, Bouso JC (2022) Significance of mammalian N,N-dimethyltryptamine (DMT): a 60-year-old debate. J Psychopharmacol 36:905–919. https://doi.org/10.1177/02698811221104054
Brito-da-Costa AM, Dias-da-Silva D, Gomes NGM et al (2020) Toxicokinetics and toxicodynamics of ayahuasca alkaloids N,N-dimethyltryptamine (DMT), harmine, harmaline and tetrahydroharmine: clinical and forensic impact. Pharmaceuticals (Basel). https://doi.org/10.3390/ph13110334
Riba J, McIlhenny EH, Valle M et al (2012) Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal 4:610–616. https://doi.org/10.1002/dta.1344
Caspar AT, Gaab JB, Michely JA et al (2018) Metabolism of the tryptamine-derived new psychoactive substances 5-MeO-2-Me-DALT, 5-MeO-2-Me-ALCHT, and 5-MeO-2-Me-DIPT and their detectability in urine studied by GC-MS, LC-MS n, and LC-HR-MS/MS. Drug Test Anal 10:184–195. https://doi.org/10.1002/dta.2197
Good M, Joel Z, Benway T et al (2023) Pharmacokinetics of N,N-dimethyltryptamine in humans. Eur J Drug Metab Pharmacokinet 48:311–327. https://doi.org/10.1007/s13318-023-00822-y
Berlowitz I, Egger K, Cumming P (2022) Monoamine oxidase inhibition by plant-derived β-carbolines; Implications for the psychopharmacology of tobacco and ayahuasca. Front Pharmacol. https://doi.org/10.3389/fphar.2022.886408
Shih JC, Chen K (2004) Regulation of MAO-A and MAO-B gene expression. Curr Med Chem 11:1995–2005. https://doi.org/10.2174/0929867043364757
Yang HY, Neff NH (1973) Beta-phenylethylamine: a specific substrate for type B monoamine oxidase of brain. J Pharmacol Exp Ther 187:365–371
PubMed CAS Google Scholar
Schoepp DD, Azzaro AJ (1981) Specificity of endogenous substrates for types A and B monoamine oxidase in rat striatum. J Neurochem 36:2025–2031. https://doi.org/10.1111/j.1471-4159.1981.tb10829.x
Ekblom J, Jossan SS, Bergström M et al (1993) Monoamine oxidase-B in astrocytes. Glia 8:122–132. https://doi.org/10.1002/glia.440080208
Cumming P (2009) Imaging dopamine. Cambridge University Press, Cambridge
Hoffman GR, Olson MG, Schoffstall AM et al (2023) Classics in chemical neuroscience: selegiline, isocarboxazid, phenelzine, and tranylcypromine. ACS Chem Neurosci 14:4064–4075. https://doi.org/10.1021/acschemneuro.3c00591
Shulman KI, Fischer HD, Herrmann N et al (2009) Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors. J Clin Psychiatry 70:1681–1686. https://doi.org/10.4088/JCP.08m05041blu
Shah NS, Hedden MP (1978) Behavioral effects and metabolic fate of N,N-dimethyltryptamine in mice pretreated with beta-diethylaminoethyl-diphenylpropylacetate (SKF 525-A), improniazid and chlorpromazine. Pharmacol Biochem Behav 8:351–356. https://doi.org/10.1016/0091-3057(78)90070-9
Morinan A, Collier JG (1981) Effects of pargyline and SKF-525A on brain N,N-dimethyltryptamine concentrations and hyperactivity in mice. Psychopharmacology 75:179–183. https://doi.org/10.1007/BF00432184
Pic-Taylor A, da Motta LG, de Morais JA et al (2015) Behavioural and neurotoxic effects of ayahuasca infusion ( Banisteriopsis caapi and Psychotria viridis ) in female Wistar rat. Behav Proc 118:102–110. https://doi.org/10.1016/J.BEPROC.2015.05.004
Durante Í, Dos Santos RG, Bouso JC, Hallak JE (2021) Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry 43:362–369. https://doi.org/10.1590/1516-4446-2020-0913
Fábregas JM, González D, Fondevila S et al (2010) Assessment of addiction severity among ritual users of ayahuasca. Drug Alcohol Depend 111:257–261. https://doi.org/10.1016/j.drugalcdep.2010.03.024
Bouso JC, Andión Ó, Sarris JJ et al (2022) Adverse effects of ayahuasca: results from the global ayahuasca survey. PLOS Global Public Health 2:e0000438. https://doi.org/10.1371/journal.pgph.0000438
Halpern JH, Sherwood AR, Passie T et al (2008) Evidence of health and safety in American members of a religion who use a hallucinogenic sacrament. Med Sci Monit 14:Sr15–Sr22
PubMed Google Scholar
Heise CW, Brooks DE (2017) Ayahuasca exposure: descriptive analysis of calls to US poison control centers from 2005 to 2015. J Med Toxicol 13:245–248. https://doi.org/10.1007/s13181-016-0593-1
Suárez Álvarez C, Mazarrasa J, Bouso JC et al (2023) Ayahuasca global consumption & reported deaths. In: ICEERS, Excutive Summary, https://www.iceers.org/ayahuasca-global-consumption-deaths/
Warren JM, Dham-Nayyar P, Alexander J (2013) Recreational use of naturally occurring dimethyltryptamine—contributing to psychosis? Aust N Z J Psychiatry 47:398–399. https://doi.org/10.1177/0004867412462749
Lewis SE (2008) Ayahuasca and spiritual crisis: liminality as space for personal growth. Anthropol Conscious 19:109–133. https://doi.org/10.1111/j.1556-3537.2008.00006.x
Labate BC, MacRae E (2016) Ayahuasca, ritual and religion in Brazil. Routledge
Londoño D, Mazarrasa J, Aixalà MB et al (2019) Towards better ayahuasca practices. A guide for organizers and participants. In: ICEERS, new guide: towards better ayahuasca practices. https://www.iceers.org/launch-of-the-guide-towards-better-ayahuasca-practices/
Szára S (1956) Dimethyltryptamin: its metabolism in man; the relation to its psychotic effect to the serotonin metabolism. Experientia 12:441–442. https://doi.org/10.1007/BF02157378
Dittrich A, Bickel P, Schöpf J, Zimmer D (1976) Comparison of altered states of consciousness induced by the hallucinogens (–)-delta9-trans-tetrahydrocannabinol (delta9-THC) and N, N-dimethyltryptamine (DMT) (author’s transl). Arch Psychiatr Nervenkr 223:77–87. https://doi.org/10.1007/BF00367455
Bickel P, Dittrich A, Schoepf J (1976) Altered states of consciousness induced by N, N-dimethyltryptamine (DMT). Pharmakopsychiatr Neuropsychopharmakol 9:220–225. https://doi.org/10.1055/s-0028-1094495
Strassman RJ (1996) Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res 73:121–124. https://doi.org/10.1016/0166-4328(96)00081-2
Callaway JC (2005) Fast and slow metabolizers of Hoasca. J Psychoactive Drugs 37:157–161. https://doi.org/10.1080/02791072.2005.10399797
Schenberg EE, Alexandre JFM, Filev R et al (2015) Acute biphasic effects of ayahuasca. PLoS One 10:e0137202. https://doi.org/10.1371/journal.pone.0137202
Li S, Zhang Y, Deng G et al (2017) Exposure characteristics of the analogous β-carboline alkaloids harmaline and harmine based on the efflux transporter of multidrug resistance protein 2. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00541
Passie T, Seifert J, Schneider U, Emrich HM (2002) The pharmacology of psilocybin. Addict Biol 7:357–364. https://doi.org/10.1080/1355621021000005937
Abramson HA, Jarvik ME, Gorin MH, Hirsch MW (1956) Lysergic acid diethylamide (LSD-25): XVII. Tolerance development and its relationship to a theory of psychosis. J Psychol 41:81–105. https://doi.org/10.1080/00223980.1956.9916206
Valle M, Maqueda AE, Rabella M et al (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26:1161–1175. https://doi.org/10.1016/j.euroneuro.2016.03.012
Keiser MJ, Setola V, Irwin JJ et al (2009) Predicting new molecular targets for known drugs. Nature 462:175–181. https://doi.org/10.1038/nature08506
Ray TS (2010) Psychedelics and the human receptorome. PLoS One 5:e9019. https://doi.org/10.1371/journal.pone.0009019
McKenna DJ, Repke DB, Lo L, Peroutka SJ (1990) Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 29:193–198. https://doi.org/10.1016/0028-3908(90)90001-8
Lyon RA, Titeler M, Seggel MR, Glennon RA (1988) Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens. Eur J Pharmacol 145:291–297. https://doi.org/10.1016/0014-2999(88)90432-3
Pierce PA, Peroutka SJ (1989) Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology 97:118–122. https://doi.org/10.1007/BF00443425
Aghajanian GK, Marek GJ (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21:16–23. https://doi.org/10.1016/S0893-133X(98)00135-3
Nichols DE (2004) Hallucinogens. Pharmacol Ther 101:131–181. https://doi.org/10.1016/j.pharmthera.2003.11.002
Smith RL, Canton H, Barrett RJ, Sanders-Bush E (1998) Agonist properties of N, N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav 61:323–330. https://doi.org/10.1016/S0091-3057(98)00110-5
Cozzi NV, Gopalakrishnan A, Anderson LL et al (2009) Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural Transm 116:1591. https://doi.org/10.1007/s00702-009-0308-8
Kaufman J, DeLorenzo C, Choudhury S, Parsey RV (2016) The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol 26:397–410. https://doi.org/10.1016/j.euroneuro.2015.12.039
Savitz J, Lucki I, Drevets WC (2009) 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 88:17–31. https://doi.org/10.1016/j.pneurobio.2009.01.009
Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED (2014) 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 231:623–636. https://doi.org/10.1007/s00213-013-3389-x
Carhart-Harris RL, Nutt DJ (2017) Serotonin and brain function: a tale of two receptors. J Psychopharmacol 31:1091–1120. https://doi.org/10.1177/0269881117725915
Stamper CE, Hassell JE, Kapitz AJ et al (2017) Activation of 5-HT1A receptors in the rat dorsomedial hypothalamus inhibits stress-induced activation of the hypothalamic-pituitary-adrenal axis. Stress 20:223–230. https://doi.org/10.1080/10253890.2017.1301426
Jaster AM, de la Fuente RM, González-Maeso J (2022) Molecular targets of psychedelic-induced plasticity. J Neurochem 162:80–88. https://doi.org/10.1111/jnc.15536
Rossi GN, Guerra LTL, Baker GB et al (2022) Molecular pathways of the therapeutic effects of ayahuasca, a botanical psychedelic and potential rapid-acting antidepressant. Biomolecules 12:1618. https://doi.org/10.3390/biom12111618
Grella B, Dukat M, Young R et al (1998) Investigation of hallucinogenic and related beta-carbolines. Drug Alcohol Depend 50:99–107. https://doi.org/10.1016/s0376-8716(97)00163-4
Grella B, Teitler M, Smith C et al (2003) Binding of β-Carbolines at 5-HT2 serotonin receptors. Bioorg Med Chem Lett 13:4421–4425. https://doi.org/10.1016/J.BMCL.2003.09.027
Hansen HD, Ettrup A, Herth MM et al (2013) Direct comparison of [(18) F]MH.MZ and [(18) F] altanserin for 5-HT2A receptor imaging with PET. Synapse 67:328–337. https://doi.org/10.1002/syn.21643
Werle I, Nascimento LMM, dos Santos ALA et al (2024) Ayahuasca-enhanced extinction of fear behaviour: role of infralimbic cortex 5-HT 2A and 5-HT 1A receptors. Br J Pharmacol. https://doi.org/10.1111/bph.16315
Chagraoui A, Thibaut F, Skiba M et al (2016) 5-HT2C receptors in psychiatric disorders: a review. Prog Neuropsychopharmacol Biol Psychiatry 66:120–135. https://doi.org/10.1016/j.pnpbp.2015.12.006
Minuzzi L, Cumming P (2010) Agonist binding fraction of dopamine D2/3 receptors in rat brain: a quantitative autoradiographic study. Neurochem Int 56:747–752. https://doi.org/10.1016/j.neuint.2010.01.010
Minuzzi L, Nomikos GG, Wade MR et al (2005) Interaction between LSD and dopamine D2/3 binding sites in pig brain. Synapse 56:198–204. https://doi.org/10.1002/syn.20141
Liu R, Wang Y, Chen X et al (2021) Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage Clin 30:102599. https://doi.org/10.1016/j.nicl.2021.102599
Wise RA, Jordan CJ (2021) Dopamine, behavior, and addiction. J Biomed Sci 28:83. https://doi.org/10.1186/s12929-021-00779-7
Glennon RA, Dukat M, Grella B et al (2000) Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend 60:121–132. https://doi.org/10.1016/s0376-8716(99)00148-9
de Castro-Neto EF, da Cunha RH, da Silveira DX et al (2013) Changes in aminoacidergic and monoaminergic neurotransmission in the hippocampus and amygdala of rats after ayahuasca ingestion. World J Biol Chem 4:141–147. https://doi.org/10.4331/wjbc.v4.i4.141
Pedersen K, Simonsen M, Østergaard SD et al (2007) Mapping the amphetamine-evoked changes in [11C]raclopride binding in living rat using small animal PET: modulation by MAO-inhibition. Neuroimage 35:38–46. https://doi.org/10.1016/j.neuroimage.2006.11.038
Jensen SB, Olsen AK, Pedersen K, Cumming P (2006) Effect of monoamine oxidase inhibition on amphetamine-evoked changes in dopamine receptor availability in the living pig: a dual tracer PET study with [11C]harmine and [11C]raclopride. Synapse 59:427–434. https://doi.org/10.1002/syn.20258
Brierley DI, Davidson C (2013) Harmine augments electrically evoked dopamine efflux in the nucleus accumbens shell. J Psychopharmacol 27:98–108. https://doi.org/10.1177/0269881112463125
Brierley DI, Davidson C (2012) Developments in harmine pharmacology–implications for ayahuasca use and drug-dependence treatment. Prog Neuropsychopharmacol Biol Psychiatry 39:263–272. https://doi.org/10.1016/J.PNPBP.2012.06.001
Liester MB, Prickett JI (2012) Hypotheses regarding the mechanisms of ayahuasca in the treatment of addictions. J Psychoactive Drugs 44:200–208. https://doi.org/10.1080/02791072.2012.704590
Francardo V, Bez F, Wieloch T et al (2014) Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain 137:1998–2014. https://doi.org/10.1093/brain/awu107
Szabó Í, Varga VÉ, Dvorácskó S et al (2021) N , N -Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology 192:108612. https://doi.org/10.1016/j.neuropharm.2021.108612
Shi C-C, Liao J-F, Chen C-F (2001) Comparative study on the vasorelaxant effects of three harmala alkaloids in vitro. Jpn J Pharmacol 85:299–305. https://doi.org/10.1254/jjp.85.299
Weiss M, Buldakova S, Dutova E (1995) Interaction of the beta-carboline harmaline with a GABA-benzodiazepine mechanism: an electrophysiological investigation on rat hippocampal slices. Brain Res 695:105–109. https://doi.org/10.1016/0006-8993(95)00630-9
Jain S, Panuganti V, Jha S, Roy I (2020) Harmine acts as an indirect inhibitor of intracellular protein aggregation. ACS Omega 5:5620–5628. https://doi.org/10.1021/acsomega.9b02375
Zhang L, Li D, Yu S (2020) Pharmacological effects of harmine and its derivatives: a review. Arch Pharm Res 43:1259–1275. https://doi.org/10.1007/s12272-020-01283-6
Li S-P, Wang Y-W, Qi S-L et al (2018) Analogous β-carboline alkaloids harmaline and harmine ameliorate scopolamine-induced cognition dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00346
Huang J, Liu Y, Chen J et al (2022) Harmine is an effective therapeutic small molecule for the treatment of cardiac hypertrophy. Acta Pharmacol Sin 43:50–63. https://doi.org/10.1038/s41401-021-00639-y
Moura DJ, Richter MF, Boeira JM et al (2007) Antioxidant properties of -carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 22:293–302. https://doi.org/10.1093/mutage/gem016
Calder AE, Hasler G (2023) Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology 48:104–112. https://doi.org/10.1038/s41386-022-01389-z
Song M, Martinowich K, Lee FS (2017) BDNF at the synapse: why location matters. Mol Psychiatry 22:1370–1375. https://doi.org/10.1038/mp.2017.144
Colucci-D’Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21:7777. https://doi.org/10.3390/ijms21207777
de Almeida RN, Galvãoda Silva ACMFS et al (2019) Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol 10:1234. https://doi.org/10.3389/fpsyg.2019.01234
Rocha JM, Rossi GN, de Lima OF et al (2021) Effects of ayahuasca on the recognition of facial expressions of emotions in naive healthy volunteers. J Clin Psychopharmacol 41:267–274. https://doi.org/10.1097/JCP.0000000000001396
Ly C, Greb AC, Cameron LP et al (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23:3170–3182. https://doi.org/10.1016/j.celrep.2018.05.022
Cameron LP, Benson CJ, Dunlap LE, Olson DE (2018) Effects of N , N -dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci 9:1582–1590. https://doi.org/10.1021/acschemneuro.8b00134
Vargas MV, Dunlap LE, Dong C et al (2023) Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science (1979) 379:700–706. https://doi.org/10.1126/science.adf0435
Cameron LP, Benson CJ, DeFelice BC et al (2019) Chronic, intermittent microdoses of the psychedelic N, N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem Neurosci 10:3261–3270. https://doi.org/10.1021/acschemneuro.8b00692
Zheng Z-H, Lin X-C, Lu Y et al (2023) Harmine exerts anxiolytic effects by regulating neuroinflammation and neuronal plasticity in the basolateral amygdala. Int Immunopharmacol 119:110208. https://doi.org/10.1016/j.intimp.2023.110208
Fortunato JJ, Réus GZ, Kirsch TR et al (2009) Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 33:1425–1430. https://doi.org/10.1016/j.pnpbp.2009.07.021
Morales-Garcia JA, Calleja-Conde J, Lopez-Moreno JA et al (2020) N, N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo. Transl Psychiatry 10:331. https://doi.org/10.1038/s41398-020-01011-0
Giacobbo BL, Doorduin J, Moraga-Amaro R et al (2020) Chronic harmine treatment has a delayed effect on mobility in control and socially defeated rats. Psychopharmacology 237:1595–1606. https://doi.org/10.1007/s00213-020-05483-2
Morales-García JA, de la Fuente RM, Alonso-Gil S et al (2017) The alkaloids of Banisteriopsis caapi , the plant source of the Amazonian hallucinogen ayahuasca, stimulate adult neurogenesis in vitro. Sci Rep 7:5309. https://doi.org/10.1038/s41598-017-05407-9
Hämmerle B, Ulin E, Guimera J et al (2011) Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling. Development 138:2543–2554. https://doi.org/10.1242/dev.066167
Riba J, Romero S, Grasa E et al (2006) Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology 186:93–98. https://doi.org/10.1007/s00213-006-0358-7
Craig A (2003) Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–505. https://doi.org/10.1016/S0959-4388(03)00090-4
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. https://doi.org/10.1038/nrn894
Hamill J, Hallak J, Dursun SM, Baker G (2019) Ayahuasca: psychological and physiologic effects, pharmacology and potential uses in addiction and mental illness. Curr Neuropharmacol 17:108–128. https://doi.org/10.2174/1570159X16666180125095902
Merkl A, Schneider G-H, Schönecker T et al (2013) Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol 249:160–168. https://doi.org/10.1016/j.expneurol.2013.08.017
Bewernick BH, Hurlemann R, Matusch A et al (2010) Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry 67:110–116. https://doi.org/10.1016/j.biopsych.2009.09.013
Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008) A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. https://doi.org/10.1002/hbm.20426
Pizzagalli DA, Holmes AJ, Dillon DG et al (2009) Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166:702–710. https://doi.org/10.1176/appi.ajp.2008.08081201
Drevets WC, Savitz J, Trimble M (2008) The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681. https://doi.org/10.1017/S1092852900013754
Daumann J, Heekeren K, Neukirch A et al (2008) Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 200:573–583. https://doi.org/10.1007/s00213-008-1237-1
Daumann J, Wagner D, Heekeren K et al (2010) Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol 24:1515–1524. https://doi.org/10.1177/0269881109103227
Gouzoulis-Mayfrank E, Heekeren K, Neukirch A et al (2006) Inhibition of return in the human 5HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology 31:431–441. https://doi.org/10.1038/sj.npp.1300882
De Araujo DB, Ribeiro S, Cecchi GA et al (2012) Seeing with the eyes shut: neural basis of enhanced imagery following ayahuasca ingestion. Hum Brain Mapp 33:2550–2560. https://doi.org/10.1002/HBM.21381
Frost JA (1999) Language processing is strongly left lateralized in both sexes: evidence from functional MRI. Brain 122:199–208. https://doi.org/10.1093/brain/122.2.199
Brewer JA, Worhunsky PD, Gray JR et al (2011) Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA 108:20254–20259. https://doi.org/10.1073/pnas.1112029108
Viol A, Palhano-Fontes F, Onias H et al (2017) Shannon entropy of brain functional complex networks under the influence of the psychedelic ayahuasca. Sci Rep 7:7388. https://doi.org/10.1038/s41598-017-06854-0
Carhart-Harris RL, Leech R, Hellyer PJ et al (2014) The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. https://doi.org/10.3389/fnhum.2014.00020
Sampedro F, de la Fuente RM, Valle M et al (2017) Assessing the psychedelic “after-glow” in ayahuasca users: post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol 20:698–711. https://doi.org/10.1093/ijnp/pyx036
Carhart-Harris RL, Muthukumaraswamy S, Roseman L et al (2016) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci 113:4853–4858. https://doi.org/10.1073/pnas.1518377113
Kometer M, Schmidt A, Jancke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. https://doi.org/10.1523/JNEUROSCI.3007-12.2013
Abdallah CG, Niciu MJ, Fenton LR et al (2014) Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom 83:298–307. https://doi.org/10.1159/000361078
Fox MD, Snyder AZ, Vincent JL et al (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci 102:9673–9678. https://doi.org/10.1073/pnas.0504136102
Carhart-Harris RL, Leech R, Erritzoe D et al (2013) Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39:1343–1351. https://doi.org/10.1093/schbul/sbs117
Pasquini L, Palhano-Fontes F, Araujo DB (2020) Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J Psychopharmacol 34:623–635. https://doi.org/10.1177/0269881120909409
Mallaroni P, Mason NL, Kloft L et al (2024) Shared functional connectome fingerprints following ritualistic ayahuasca intake. Neuroimage 285:120480. https://doi.org/10.1016/j.neuroimage.2023.120480
Yeo BTT, Krienen FM, Sepulcre J et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. https://doi.org/10.1152/jn.00338.2011
Timmermann C, Roseman L, Haridas S et al (2023) Human brain effects of DMT assessed via EEG-fMRI. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2218949120
Huntenburg JM, Bazin P-L, Margulies DS (2018) Large-scale gradients in human cortical organization. Trends Cogn Sci 22:21–31. https://doi.org/10.1016/j.tics.2017.11.002
Arruda Sanchez T, Ramos LR, Araujo F et al (2024) Emotion regulation effects of ayahuasca in experienced subjects during implicit aversive stimulation: an fMRI study. J Ethnopharmacol 320:117430. https://doi.org/10.1016/j.jep.2023.117430
Don NS, McDonough BE, Moura G et al (1998) Effects of ayahuasca on the human EEG. Phytomedicine 5:87–96. https://doi.org/10.1016/S0944-7113(98)80003-2
Riba J, Anderer P, Morte A et al (2002) Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol 53:613–628. https://doi.org/10.1046/j.1365-2125.2002.01609.x
Riba J, Anderer P, Jané F et al (2004) Effects of the south american psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology 50:89–101. https://doi.org/10.1159/000077946
Saletu B, Barbanoj MJ, Anderer P et al (1993) Clinical-pharmacological study with the two isomers (d-, l-) of fenfluramine and its comparison with chlorpromazine and d-amphetamine: blood levels, EEG mapping and safety evaluation. Methods Find Exp Clin Pharmacol 15:291–312
Herrmann W (1986) Pharmaco-EEG: computer EEG analysis to describe the projection of drug effects on a functional cerebral level in humans. In: Lopes da Silva FH, Storm van Leeuwen W, Rémond A (eds) Clinical applications of computer analysis of EEG and other neurophysiological signals. Elsevier, Amsterdam, pp 385–448
Kłodzinska A, Bijak M, Tokarski K, Pilc A (2002) Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav 73:327–332. https://doi.org/10.1016/S0091-3057(02)00845-6
Alonso JF, Romero S, Mañanas MÀ, Riba J (2015) Serotonergic psychedelics temporarily modify information transfer in humans. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyv039
Heekeren K, Daumann J, Neukirch A et al (2008) Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 199:77–88. https://doi.org/10.1007/s00213-008-1129-4
Vogel EK, Luck SJ (2000) The visual N1 component as an index of a discrimination process. Psychophysiology 37:190–203
Umbricht D, Vollenweider FX, Schmid L et al (2003) Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology 28:170–181. https://doi.org/10.1038/sj.npp.1300005
Umbricht D, Koller R, Schmid L et al (2003) How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry 53:1120–1131. https://doi.org/10.1016/S0006-3223(02)01642-6
Timmermann C, Roseman L, Schartner M et al (2019) Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep 9:16324. https://doi.org/10.1038/s41598-019-51974-4
Alamia A, Timmermann C, Nutt DJ et al (2020) DMT alters cortical travelling waves. Elife. https://doi.org/10.7554/eLife.59784
Alamia A, VanRullen R (2019) Alpha oscillations and traveling waves: signatures of predictive coding? PLoS Biol 17:e3000487. https://doi.org/10.1371/journal.pbio.3000487
Schartner MM, Timmermann C (2020) Neural network models for DMT-induced visual hallucinations. Neurosci Conscious. https://doi.org/10.1093/NC/NIAA024
Pallavicini C, Cavanna F, Zamberlan F et al (2021) Neural and subjective effects of inhaled N, N-dimethyltryptamine in natural settings. J Psychopharmacol 35:406–420. https://doi.org/10.1177/0269881120981384
Tagliazucchi E, Zamberlan F, Cavanna F et al (2021) Baseline power of theta oscillations predicts mystical-type experiences induced by DMT in a natural setting. Front Psychiatry. https://doi.org/10.3389/fpsyt.2021.720066
Moosmann M, Ritter P, Krastel I et al (2003) Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 20:145–158. https://doi.org/10.1016/S1053-8119(03)00344-6
Buchsbaum MS, Kessler R, King A et al (1984) Simultaneous cerebral glucography with positron emission tomography and topographic electroencephalography. Prog Brain Res 62:263–269. https://doi.org/10.1016/S0079-6123(08)62182-2
de Munck JC, Gonçalves SI, Huijboom L et al (2007) The hemodynamic response of the alpha rhythm: an EEG/fMRI study. Neuroimage 35:1142–1151. https://doi.org/10.1016/j.neuroimage.2007.01.022
Başar E, Güntekin B (2009) Darwin’s evolution theory, brain oscillations, and complex brain function in a new “Cartesian view.” Int J Psychophysiol 71:2–8. https://doi.org/10.1016/j.ijpsycho.2008.07.018
Klimesch W (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. https://doi.org/10.1016/j.tics.2012.10.007
Başar E, Yordanova J, Kolev V, Başar-Eroglu C (1997) Is the alpha rhythm a control parameter for brain responses? Biol Cybern 76:471–480. https://doi.org/10.1007/s004220050360
Mayer A, Schwiedrzik CM, Wibral M et al (2016) Expecting to see a letter: alpha oscillations as carriers of top-down sensory predictions. Cereb Cortex 26:3146–3160. https://doi.org/10.1093/cercor/bhv146
Wang L, Hagoort P, Jensen O (2018) Language prediction is reflected by coupling between frontal gamma and posterior alpha oscillations. J Cogn Neurosci 30:432–447. https://doi.org/10.1162/jocn_a_01190
Carhart-Harris RL, Friston KJ (2019) REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev 71:316–344. https://doi.org/10.1124/pr.118.017160
Vollenweider FX, Preller KH (2020) Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci 21:611–624. https://doi.org/10.1038/s41583-020-0367-2
Vollenweider FX, Geyer MA (2001) A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 56:495–507. https://doi.org/10.1016/s0361-9230(01)00646-3
Corlett PR, Horga G, Fletcher PC et al (2019) Hallucinations and strong priors. Trends Cogn Sci 23:114–127. https://doi.org/10.1016/j.tics.2018.12.001
Doss MK, Madden MB, Gaddis A et al (2022) Models of psychedelic drug action: modulation of cortical-subcortical circuits. Brain 145:441–456. https://doi.org/10.1093/brain/awab406
Erritzoe D, Timmermann C, Godfrey K et al (2024) Exploring mechanisms of psychedelic action using neuroimaging. Nat Mental Health 2:141–153. https://doi.org/10.1038/s44220-023-00172-3
Kwan AC, Olson DE, Preller KH, Roth BL (2022) The neural basis of psychedelic action. Nat Neurosci. https://doi.org/10.1038/s41593-022-01177-4
Gattuso JJ, Perkins D, Ruffell S et al (2023) Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol 26:155–188. https://doi.org/10.1093/ijnp/pyac074
McCulloch DE-W, Knudsen GM, Barrett FS et al (2022) Psychedelic resting-state neuroimaging: a review and perspective on balancing replication and novel analyses. Neurosci Biobehav Rev 138:104689. https://doi.org/10.1016/j.neubiorev.2022.104689
Bouso JC, González D, Fondevila S et al (2012) Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: a longitudinal study. PLoS One 7:e42421. https://doi.org/10.1371/journal.pone.0042421
Elkins DN, Hedstrom LJ, Hughes LL et al (1988) Toward a humanistic-phenomenological spirituality. J Humanist Psychol 28:5–18. https://doi.org/10.1177/0022167888284002
Bouso JC, Palhano-Fontes F, Rodriguez-Fornells A et al (2015) Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur Neuropsychopharmacol 25:483–492. https://doi.org/10.1016/j.euroneuro.2015.01.008
Simonsson O, Bouso JC, Kurth F et al (2022) Preliminary evidence of links between ayahuasca use and the corpus callosum. Front Psychiatry. https://doi.org/10.3389/fpsyt.2022.1002455
Meyer B, Röricht S, Woiciechowsky C (1998) Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369. https://doi.org/10.1002/ana.410430314
Petrov D, Mansfield C, Moussy A, Hermine O (2017) ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front Aging Neurosci. https://doi.org/10.3389/fnagi.2017.00068
Seidlitz J, Nadig A, Liu S et al (2020) Transcriptomic and cellular decoding of regional brain vulnerability to neurogenetic disorders. Nat Commun 11:3358. https://doi.org/10.1038/s41467-020-17051-5
Mallaroni P, Mason NL, Kloft L et al (2023) Cortical structural differences following repeated ayahuasca use hold molecular signatures. Front Neurosci 17:1217079. https://doi.org/10.3389/fnins.2023.1217079
Smith DA, Bailey JM, Williams D, Fantegrossi WE (2014) Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J Pharmacol Exp Ther 351:485–491. https://doi.org/10.1124/jpet.114.219337
Bouso JC, Fábregas JM, Antonijoan RM et al (2013) Acute effects of ayahuasca on neuropsychological performance: differences in executive function between experienced and occasional users. Psychopharmacology 230:415–424. https://doi.org/10.1007/s00213-013-3167-9
Miller L, Bansal R, Wickramaratne P et al (2014) Neuroanatomical correlates of religiosity and spirituality: a study in adults at high and low familial risk for depression. JAMA Psychiat 71:128–135. https://doi.org/10.1001/jamapsychiatry.2013.3067
Wall MB, Harding R, Zafar R et al (2023) Neuroimaging in psychedelic drug development: past, present, and future. Mol Psychiatry 28:3573–3580. https://doi.org/10.1038/s41380-023-02271-0
Gorgolewski KJ, Auer T, Calhoun VD et al (2016) The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data 3:160044. https://doi.org/10.1038/sdata.2016.44
Esteban O, Markiewicz CJ, Blair RW et al (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. https://doi.org/10.1038/s41592-018-0235-4
Raval NR, Johansen A, Donovan LL et al (2021) A single dose of psilocybin increases synaptic density and decreases 5-HT2A receptor density in the pig brain. Int J Mol Sci. https://doi.org/10.3390/ijms22020835
Poulie CBM, Jensen AA, Halberstadt AL, Kristensen JL (2020) DARK classics in chemical neuroscience: NBOMes. ACS Chem Neurosci 11:3860–3869. https://doi.org/10.1021/acschemneuro.9b00528
Heim R (2004) Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts. Doctoral Dissertation, Freie Universität Berlin
Cumming P, Wong DF, Gillings N et al (2002) Specific binding of [(11)C]raclopride and N-[(3)H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J Cereb Blood Flow Metab 22:596–604. https://doi.org/10.1097/00004647-200205000-00011
Fowler JS, Logan J, Wang G-J et al (2005) Comparison of monoamine oxidase a in peripheral organs in nonsmokers and smokers. J Nucl Med 46:1414–1420
Rodd R (2002) Snuff synergy: preparation, use and pharmacology of Yopo and Banisteriopsis Caapi among the piaroa of southern Venezuela. J Psychoactive Drugs 34:273–279. https://doi.org/10.1080/02791072.2002.10399963
Download references
Open access funding provided by University of Zurich. PC received grant support from the Swiss National Science Foundation (Grant Number 320030-204978).
Author information
Authors and affiliations.
Department of Adult Psychiatry and Psychotherapy, Psychiatric University Clinic Zurich and University of Zurich, Zurich, Switzerland
Klemens Egger, Helena D. Aicher & Milan Scheidegger
Neuroscience Center Zurich, University of Zurich and Swiss Federal Institute of Technology Zurich, Zurich, Switzerland
Department of Nuclear Medicine, Bern University Hospital, Bern, Switzerland
Klemens Egger & Paul Cumming
Department of Psychology, University of Zurich, Zurich, Switzerland
Helena D. Aicher
School of Psychology and Counselling, Queensland University of Technology, Brisbane, Australia
Paul Cumming
You can also search for this author in PubMed Google Scholar
Contributions
KE: writing—original draft, writing—review and editing; visualization. HA: writing—original draft, writing—review and editing. PC: writing—original draft, writing—review and editing. MS: writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Klemens Egger .
Ethics declarations
Conflict of interest.
MS co-founded Reconnect Labs, an academic spin-off at the University of Zurich. KE, HDA and PC have no relevant financial or non-financial interests to disclose.
Ethical approval and consent to participate
Consent to publish, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 243 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Egger, K., Aicher, H.D., Cumming, P. et al. Neurobiological research on N,N -dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors. Cell. Mol. Life Sci. 81 , 395 (2024). https://doi.org/10.1007/s00018-024-05353-6
Download citation
Received : 16 April 2024
Revised : 19 June 2024
Accepted : 04 July 2024
Published : 10 September 2024
DOI : https://doi.org/10.1007/s00018-024-05353-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Psychedelics
- β-carbolines
- MAO inhibition
Advertisement
- Find a journal
- Publish with us
- Track your research
Important notice
This announcement does not contain or constitute an offer of, or the solicitation of an offer to buy or subscribe for, any securities. There will be no public offer of the securities in any jurisdiction. Neither this announcement nor anything contained herein shall form the basis of, or be relied upon in connection with, any offer or commitment whatsoever in any jurisdiction. An investment decision regarding the securities referred to herein should only be made on the basis of the securities prospectus.
This announcement is an advertisement and does not, under any circumstances, constitute a public offering or an invitation to the public in connection with any offer within the meaning of Regulation (EU) 2017/1129. The final prospectus, when published, will be available on the website of the Luxembourg Stock Exchange ( www.luxse.com ).
The securities referred to herein will not be registered under the U.S. Securities Act of 1933, as amended (the "U.S. Securities Act"), or any U.S. State security laws and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements under the U.S. Securities Act or to, or for the benefit of, U.S. persons.
The tender offer referenced herein is not being made, directly or indirectly, in or into the United States by use of the mails or by any means or instrumentality (including, without limitation, e-mail, facsimile transmission, telephone and the internet) of interstate or foreign commerce, or of any facility of a national securities exchange of the United States and the tender offer cannot be accepted by any such use, means, instrumentality or facility or from within the United States.
Viewing the materials you seek to access may not be lawful in certain jurisdictions. In other jurisdictions, only certain categories of person may be allowed to view such materials. Any person who wishes to view these materials must first satisfy themselves that they are not subject to any local requirements that prohibit or restrict them from doing so.
If you are not permitted to view materials on this webpage or are in any doubt as to whether you are permitted to view these materials, please exit this webpage.
Basis of access
Access to electronic versions of these materials is being made available on this webpage by Bayer in good faith and for information purposes only. Making press announcements and other documents available in electronic format on this webpage does not constitute an offer to sell or the solicitation of an offer to buy securities issued by Bayer. Further, it does not constitute a recommendation by Bayer, or any other party to buy or sell securities issued by Bayer.
Confirmation of understanding and acceptance of disclaimer
By clicking on the “I AGREE” button, I certify that I am not located in the United States, Australia, Canada, South Africa or Japan or any other jurisdiction, where access to the materials is prohibited or restricted.
I have read and understood the disclaimer set out above. I understand that it may affect my rights. I agree to be bound by its terms. By clicking on the “I AGREE” button, I confirm that I am permitted to proceed to electronic versions of these materials.
Please confirm your location here:
Disclaimer – important.
The following materials are not directed at or to be accessed by persons located in the United States, Australia, Canada or Japan. These materials do not constitute or form a part of any offer or solicitation to purchase or subscribe for securities in the United States, Australia, Canada or Japan or in any other jurisdiction in which such offer or solicitation is not authorized or to any person to whom it is unlawful to make such offer or solicitation.
The securities mentioned herein have not been, and will not be, registered under the Securities Act and may not be offered or sold in the United States, except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act. There will be no public offer of the securities in the United States.
In the United Kingdom the following materials are only directed at (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth companies, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on the materials or any of their contents.
In relation to each member state of the European Economic Area which has implemented the Directive 2003/71/EC, and any amendments thereto (the “Prospectus Directive”)(each a “Relevant Member State”), an offer to the public of the securities has not been made and will not be made in such Relevant Member State, except that an offer to the public in such Relevant Member State of any securities may be made at any time under the following exemptions from the Prospectus Directive, if they have been implemented in the Relevant Member State:
- to any legal entity which is a qualified investor as defined in the Prospectus Directive,
- to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, or
- in any other circumstances falling within Article 3(2) of the Prospectus Directive;
provided that no such offer shall result in a requirement to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase any securities, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State, and the expression “Prospectus Directive” includes any relevant implementing measure in each Relevant Member State.
By clicking on the “I AGREE” button, I certify that I am not located in the United States, Australia, Canada or Japan or any other jurisdiction, where access to the materials is prohibited or restricted.
Important Notice
The securities referred to herein will not be registered under the U.S. Securities Act of 1933, as amended (the "U.S. Securities Act" ), or any U.S. State security laws and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements under the U.S. Securities Act or to, or for the benefit of, U.S. persons.
This announcement does not contain or constitute an offer of, or the solicitation of an offer to buy or subscribe for, any securities. There will be no public offer of the securities in any jurisdiction. Neither this announcement nor anything contained herein shall form the basis of, or be relied upon in connection with, any offer or commitment whatsoever in any jurisdiction.
The securities referred to herein will not be registered under the U.S. Securities Act of 1933, as amended (the "U.S. Securities Act"), or any U.S. State security laws and may not be offered or sold in the United States or to, or for the benefit of, U.S. persons absent registration or an applicable exemption from the registration requirements under the U.S. Securities Act.
This website is intended to provide information to an international audience outside the USA and UK. Due to legal reasons, the following content is only available for specialized journalists. To access these pages, please confirm that you are a medical journalist and that you would like to accredit to the Bayer press portal.
This website is intended to provide information to an international audience outside the UK. Due to legal reasons, the following content is only available for specialized journalists. To access these pages, please confirm that you are a medical journalist and that you would like to accredit to the Bayer press portal.

Data from Phase III study OASIS 3 support efficacy and long-term safety of investigational compound elinzanetant in the treatment of moderate to severe vasomotor symptoms associated with menopause
Not intended for uk media – late-breaking data from phase iii long-term study oasis 3 presented at 2024 tms annual meeting:.
Results from the Phase III OASIS 3 study further support efficacy data and sustained safety data of elinzanetant over 52 weeks, adding to the positive results of the OASIS 1 and 2 studies / The long-term safety was consistent with previous findings, further confirming the favorable safety profile; no incidences of endometrial hyperplasia or endometrial malignant neoplasms; no signal of hepatotoxicity was observed / These late-breaking data will be presented for the first time at the 2024 annual meeting of The Menopause Society (TMS) / Elinzanetant is the first dual neurokinin-1 and 3 (NK-1 and 3) receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily
Berlin, September 10, 2024 –Bayer will present detailed results from the Phase III study OASIS 3, providing supporting efficacy data and sustained safety data over 52 weeks for the investigational compound elinzanetant, adding to the positive results of the OASIS 1 and 2 studies. These data will be presented at the 2024 annual meeting of The Menopause Society (TMS) taking place from September 10-14, in Chicago, IL, USA.
OASIS 3 is a Phase III efficacy and long-term safety study of elinzanetant for the treatment of vasomotor symptoms (VMS, also known as hot flashes) associated with menopause.
The data to be presented shows that elinzanetant successfully met the primary endpoint demonstrating a statistically significant reduction in the frequency of moderate to severe vasomotor symptoms from baseline to week 12 compared to placebo. Improvements in the elinzanetant arm were also seen in key secondary endpoints with a mean change from baseline in sleep disturbances and menopause related quality of life as shown by the total score over time.
There was no minimum threshold of moderate to severe VMS for inclusion in the study, and at baseline women had a mean of 6.7 VMS ( standard deviation , SD 7.2) in the elinzanetant group and 6.8 in the placebo group (SD 6.2). After 12 weeks of treatment, the number of VMS was reduced to 1.6 (SD 2.5) in the elinzanetant group and 3.4 (SD 4.2) in the placebo group. The VMS reductions were maintained throughout the study duration. In addition, improvements were seen in measures of sleep disturbances and menopause-related quality of life with elinzanetant use over 52 weeks, based on descriptive analyses.
“To see the tolerability of elinzanetant in OASIS 3 remain consistent with earlier studies is very promising,” said James A. Simon, M.D., clinical professor of obstetrics and gynecology at The George Washington University School of Medicine, Founder of IntimMedicine Specialists. “With a longer study duration and in a population with a VMS profile potentially more representative of that seen in clinical practice suggests, if approved, elinzanetant may be another option for women suffering from moderate to severe VMS.”
The safety profile of elinzanetant over 52 weeks was favorable and consistent with findings from the 26-week OASIS 1 and OASIS 2 studies, which were recently published in the Journal of the American Medical Association (JAMA) 1 . In OASIS 3, headache and COVID-19 were the most frequently reported treatment emergent adverse events (TEAEs) within the elinzanetant group. No incidences of endometrial hyperplasia, endometrial malignant neoplasms, and no liver toxicity signal were observed with elinzanetant.
“The detailed results of OASIS 3 are complementing the positive results of OASIS 1 and 2, addressing the question on its long-term profile and indicating the potential of elinzanetant to treat moderate to severe VMS associated with menopause,” said Dr. Christian Rommel, Global Head of Research and Development at Bayer AG’s Pharmaceuticals Division. “We look forward to continuing our discussions with health authorities around the world regarding marketing authorizations for this compound.”
Elinzanetant is the first dual neurokinin-1 and -3 (NK-1 and 3) receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily.
OASIS 3 (NCT05030584) is the third Phase III study in the OASIS clinical development program with positive results. Results from the first two Phase III studies OASIS 1 and 2 (NCT05042362 and NCT05099159) were recently published in the Journal of the American Medical Association (JAMA) . Based on positive results from OASIS 1, 2 and 3, Bayer has submitted a New Drug Application to the U.S. FDA for elinzanetant for the treatment of moderate to severe VMS associated with menopause. Bayer is continuing to submit applications for marketing authorizations of elinzanetant to further health authorities as well.
About the OASIS 1, 2 and 3 studies OASIS 1 and 2 (NCT05042362 and NCT05099159) are double-blind, randomized, placebo-controlled multicenter studies investigating the efficacy and safety of elinzanetant administered orally once daily in women with moderate to severe VMS associated with menopause over 26 weeks. OASIS 1 and 2 randomized 396 and 400 postmenopausal women between 40 and 65 years across 184 sites in 15 countries. Patients in the elinzanetant arm received a 120 mg dose of elinzanetant once daily for 26 weeks and patients in the control arm received a matching placebo once daily for 12 weeks, followed by elinzanetant 120 mg dose for 14 weeks. OASIS 3 (NCT05030584) is a double-blind, randomized, placebo-controlled multicenter study to investigate the efficacy and safety of elinzanetant for the treatment of vasomotor symptoms over 52 weeks in postmenopausal women. OASIS 3 randomized 628 postmenopausal women between 40 and 65 years across 83 sites in 9 countries.
About the Elinzanetant clinical development program The Phase III clinical development program of elinzanetant, OASIS, currently comprises four Phase III studies: OASIS 1, 2, 3 and 4. The OASIS 1, 2 and 3 studies investigate the efficacy and safety of elinzanetant 120 mg in women with moderate to severe VMS associated with menopause. The OASIS 4 study is an expansion of the clinical Phase III program and investigates the efficacy and safety of elinzanetant in women with moderate to severe VMS caused by endocrine therapy for treatment or prevention of breast cancer.
The design and dosing of the Phase III clinical development program is based on the positive data from two Phase II studies (RELENT-1 and SWITCH-1). RELENT-1 was a Phase Ib/IIa study investigating the safety, pharmacokinetics and preliminary efficacy of elinzanetant. SWITCH-1 was a Phase IIb study investigating the efficacy and safety of four different doses of elinzanetant compared to placebo in women with VMS.
In addition to the OASIS program, Bayer is conducting NIRVANA (NCT06112756), an exploratory Phase II randomized, parallel-group treatment, double-blind study. The primary objective is to explore the efficacy of elinzanetant on sleep disturbances associated with menopause (SDM) as determined by polysomnography (PSG). PSG is a validated method to study sleep and underlying causes of sleep disturbances. Additional objectives include exploring the efficacy of elinzanetant on SDM as determined by patient-reported outcomes and further evaluating the safety of elinzanetant.
About Elinzanetant Elinzanetant is the first dual neurokinin-1 and 3 (NK-1 and 3) receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily. Elinzanetant may address moderate to severe VMS by modulating a group of estrogen sensitive neurons in the hypothalamus region of the brain (the KNDy neurons) which, with the decrease of estrogen, become hypertrophic and lead to a hyperactivation of the thermoregulatory pathway, consequently disrupting body heat control mechanisms resulting in VMS. Based on key secondary endpoints of OASIS 1 and 2, Elinzanetant may also decrease sleep disturbances associated with menopause.
About Vasomotor Symptoms Vasomotor symptoms (VMS; also referred to as hot flashes) result from hyperactivation of the thermoregulatory pathway mediated by hypertrophy of the KNDy neurons. This is due to a decrease of estrogen, which can result from the progressive reduction of ovarian function due to natural menopause or medical intervention by bilateral oophorectomy or endocrine therapy.
VMS are reported by up to 80% of women at some point during the menopausal transition and are one of the leading causes for seeking medical attention during this phase of a woman’s life. Over one-third of menopausal women report severe symptoms, which can last 10 years or more after the last menstrual period, with relevant impact on quality of life.
VMS may also be caused by endocrine therapy, for the treatment or prevention of breast cancer, impacting quality of life and treatment adherence. For these women, there are currently no approved treatment options.
About Menopause By 2030, the global population of women experiencing menopause is projected to increase to 1.2 billion, with 47 million women entering this phase each year. Menopause is a transitional phase in women’s lives, related to the progressive decline of ovarian function. It usually occurs in women during their 40s or early 50s. It can also be the result of surgical or medical treatment such as breast cancer treatment. The hormonal decline can lead to various symptoms which can substantially affect a woman’s health, quality of life, healthcare utilization and work productivity. The most frequently reported and disruptive symptoms during the menopausal transition are VMS, sleep disturbances and mood changes. Addressing these symptoms is key to maintaining functional ability and quality of life in menopause which is highly relevant from both a healthcare and socio-economic perspective.
About Women’s Healthcare at Bayer Women’s Health is in Bayer’s DNA. As a global leader in women’s healthcare Bayer has a long-standing commitment to delivering science for a better life by advancing a portfolio of innovative treatments. Bayer offers a wide range of effective short- and long-acting birth control methods as well as therapies for menopause management and gynecological diseases. Bayer is also focusing on innovative options to address the unmet medical needs of women worldwide and to broadening treatment choices such as in menopause. Additionally, Bayer intends to provide 100 million women per year in low-and-middle income countries by 2030 with access to family planning by funding multi-stakeholder aid programs for capacity building and by ensuring the supply of affordable modern contraceptives. This is part of the comprehensive sustainability measures and commitments from 2020 onwards and in line with the Sustainable Development Goals of the United Nations.
About Bayer Bayer is a global enterprise with core competencies in the life science fields of health care and nutrition. In line with its mission, “Health for all, Hunger for none,” the company’s products and services are designed to help people and the planet thrive by supporting efforts to master the major challenges presented by a growing and aging global population. Bayer is committed to driving sustainable development and generating a positive impact with its businesses. At the same time, the Group aims to increase its earning power and create value through innovation and growth. The Bayer brand stands for trust, reliability and quality throughout the world. In fiscal 2023, the Group employed around 100,000 people and had sales of 47.6 billion euros. R&D expenses before special items amounted to 5.8 billion euros. For more information, go to www.bayer.com .
1 https://jamanetwork.com/journals/jama/fullarticle/2822766
Find more information at https://pharma.bayer.com/ Follow us on Facebook: http://www.facebook.com/bayer Follow us on LinkedIn: Bayer | Pharmaceuticals
Forward-Looking Statements This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer’s public reports which are available on the Bayer website at www.bayer.com . The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments.
Contact for global media inquiries: Katja Wiggers, phone +49 30 221541614 Email: [email protected]
Contact for US media inquiries: Courtney Ambrosi, phone 1 (908) 798-1107 Email: [email protected]
Contact for investor inquiries: Bayer Investor Relations Team, phone +49 214 30-72704 Email: [email protected] www.bayer.com/en/investors/ir-team
Sign up for our Newsletter
We will keep you informed about the latest news..

IMAGES
VIDEO
COMMENTS
Research has been conducted on the intervention of media in cases under trial. The literature indicates that trial by media is a dynamic process through which people are exposed to public opinion ...
"Trial by Media" or "Media Trial" The term "media trial" pertains to the coverage of a case, either civil or criminal, before and during the trial, which has the potential to bias the fair trial of the defendant4. It pertains to the public discussion of the merits of legal cases by the media, which is separate from reporting on
Trial by media is a phrase popular in the late 20th century and early 21st century to describe the impact of television and newspaper coverage on a person's reputation by creating a widespread perception of guilt or innocence before, or after, a verdict in a court of law. In recent times there have been numerous instances in which media has conducted the trial of an accused and has passed the ...
SUBMIT PAPER. Crime, Media, Culture: An International Journal ... of law, they were heard, through the media, by the court of public opinion. This article considers to what extent a 'trial by media' might have the potential to provide a parallel justice forum. ... (eds) The Sage Handbook of Criminological Research Methods. London: SAGE, pp ...
This research seeks to explore the role of media trials in judicial proceedings and analyze how they impact in the administration of justice. This was made possible through the use of qualitative research, specifically documentary analysis using secondary data acquired from the study's literature. Findings show that media trials interfere with the
The media's role in influencing trials in India came to attention during the Jessica Lal homicide trial. The media's role was also discussed in the case of Priyadarshini Mattoo. There have been several cases in which the media has been blamed for influencing the court judgment. Media trial is an unwarranted interference in the justice
media trials, encompassing the function of media in criminal proceedings, the tendency towards sensationalism and partiality frequently linked to media reporting, and the impact of media on the collective viewpoint. The present research paper aims to examine the negative consequences of media trials on the fundamental principle of
1International Journal of Research and Analytical Reviews Research Paper the administration of justice amount to criminal contempt ... The Media trial has become an acute problem with the ever expanding role of media. The phenomenal growth in technology ensures quick flow of information. The word, 'trial' has been defined as a formal ...
In other words, the main purpose of this paper is an endeavour to analyze the impacts of media trials to reach some conclusions which will ultimately aid in the administration of justice in ...
Because they control the minds of the masses." Malcolm X. This quotation speaks itself the gravity and concrete nature of media in democratic set-up of any nation. Present article will focus on current operational interference of media in administration of justice, the role of Supreme Court and its power under constitution.
Keywords - Media , Law, Supreme court, Fair trial 1.INTRODUCTION ―The tension between the COWIS and the media revolves around two general concerns. The first is that there should be no ‗trial by media', and the second is that it is not for the press or anyone else to ‗prejudge' a case. Justice demands that people
CERTIFICATE. This is to certify that the dissertation submitted by Manasvika S (Reg. No. 1550002) titled 'A Critical Study On Trial By Media With Special Reference To Right To Fair Trial' is a record of research work done by her during the academic year 2015-2016 under my supervision in partial fulfillment for the award of Master of ...
Riley Moran Tulane University, New Orleans, Louisiana, USA. ÒÏ. Abstract: In 2008, 22-year-old Casey Anthony was charged with first degree murder of her toddler, Caylee Anthony. Press coverage and social media discussion of the case sparked nationwide attention. Though Anthony's trial began in 2011, media platforms offered interpretations ...
media trial in India. The research will be dealing with Article 19 of the Constitution of India which states the freedom of speech and expression which includes freedom of the press which hinders with the right of an adversarial legal system in place as well as obstructs the ... the paper is to bring forward the negative impact of the media and ...
615. Media Trials: An Analysis of Ethical Issues. Jishnu D. Doctoral Scholar. Department of Media and Communication. Central University of Tamil Nadu, Thiruvarur, India. Abstract : In the ...
Media gives positive as well as negative impacts in the society. As far as administration of justice is concerned, Media interferes in the domain of judiciary. The basic principle of criminal jurisprudence is jeopardized. The Researcher in the present paper highlights the legal issues involved in media trial in India.
Previous research has linked extensive news media coverage of crimes and the criminal process to pretrial jury bias against defendants. Most research, however, has tested the effects of reading fab...
In LIC v. Manubbai Shah2, the Supreme Court reiterated that the freedom of speech and expression must be broadly construed to include the freedom to circulate one's views by word of mouth, or in writing, or through audio visual media. This includes the right to propagate one's views through the print or other media.
The Shodhganga@INFLIBNET Centre provides a platform for research students to deposit their Ph.D. theses and make it available to the entire scholarly community in open access. ... net/10603/229557. Title: Trial by media and its impact upon judicial trial a critical study: Researcher: Mittal, A K: Guide(s): singh, Anand: Keywords: Social ...
This Research paper will also throw light on the rule of sub-judice and how it is related to Media Trials. The author will further also show the other side of the study, which shows that how media trial can also help the courts in speedy trial. IndexTerms - Constitution, Free Speech and Expression, Judiciary, Democracy, Media Trials.
The most reckoning research on the positive and negative aspects of media trial has been elaborated in 200th report of the Law Commission entitled Trial by Media: Free Speech vs. Fair Trial Under Criminal Procedure (Amendments to the Contempt of Court Act, 1971) that has made recommendations to address the damaging effect of sensationalized ...
The media pushes people to prejudge the verdicts of criminal proceedings. Some people use the media to influence court case outcomes. In media trials, the media serves as a conveyor for popular sentiment. The media are also used to practice parallel elements of justice outside the confines of the courtroom.
The extraordinary media coverage regarding the disappearance of the British 3-year-old Madeleine McCann emerges as an illustrative example of a 'public drama' and 'trial by media'. This article presents a comparative analysis of the perspectives and narrative devices employed by two Portuguese newspapers in establishing a dialogue with ...
One hundred forty-four participants were randomly allocated into the study and control groups. The study group received 20 min of IFC therapy (carrier frequency: 4000 Hz, beat frequency: 100 Hz) five times per week for three weeks, while the control group received sham IFC following the same protocol, followed by 10 min of exercise in both groups.
A PULSAR Phase 3 Trial Post-Hoc Analysis: Evaluating the timing and magnitude of control of disease activity with aflibercept 8 mg and faricimab, applying similar disease activity criteria across different pivotal Phase 3 trials for nAMD; o Oral presentation in free paper session 5: AMD o Thursday, 19 September 2024; 15:18-15:24 CEST
The potent hallucinogen N,N-dimethyltryptamine (DMT) has garnered significant interest in recent years due to its profound effects on consciousness and its therapeutic psychopotential. DMT is an integral (but not exclusive) psychoactive alkaloid in the Amazonian plant-based brew ayahuasca, in which admixture of several β-carboline monoamine oxidase A (MAO-A) inhibitors potentiate the activity ...
Disorders linked to increased body weight are on the rise and obesity is a global epidemic associated with a rising risk for developing comorbidities, such as hypertension or type 2 diabetes. There is a significant need to develop a multimodal approach targeting obesity within clinical medicine. Pharmacological options to produce weight loss have been a popular research area and the novel ...
Berlin, September 10, 2024 -Bayer will present detailed results from the Phase III study OASIS 3, providing supporting efficacy data and sustained safety data over 52 weeks for the investigational compound elinzanetant, adding to the positive results of the OASIS 1 and 2 studies.These data will be presented at the 2024 annual meeting of The Menopause Society (TMS) taking place from September ...