Sign Up for our Monthly Celiac Newsletter!

Mobile menu
Celiac disease research.
The world of celiac disease research changes and evolves each and every day. Beyond Celiac keeps the community up-to-date on research in many ways, including our Research News Feed and the Research Opt-In.
Celiac Disease Research News
Potential drug to treat celiac disease prevents damage caused by gluten in recent study of molecular action.
A new study that analyzed the activity of more than 10,000 genes found that a drug being investigated to treat celiac disease prevented intestinal damage caused by gluten.
When blood tests results are highly positive, a biopsy might not be needed to diagnose celiac disease
Studies of both children and adults suggest that highly positive TTG-IgA test results might be enough for a celiac disease diagnosis.
The connection between celiac disease and obesity explored at DDW
Obesity was more common in those with celiac disease compared to those without celiac disease, analysis of a large national database of health information found.
Gluten challenges for people on a gluten-free diet seeking a celiac disease diagnosis may need to be increased, study suggests
Increased amounts of gluten in a gluten challenge for those on a gluten-free diet may be needed for accurate celiac disease blood tests done as part of diagnosis.
More than half of children with celiac disease don’t get recommended follow-up blood tests, study shows
More than half of children with celiac disease did not get recommended blood test follow-ups in a study that used artificial intelligence to scan electronic health records.
View All Research News
Celiac Disease Drug Development
- Learn about the Beyond Celiac Science Plan focused on treatments toward a cure by 2030.
- Learn about the Drug Discovery & Development Processes to understand how celiac disease medications are created
- View our interactive Drug Development Pipeline to see the current status of celiac disease drugs that are currently in development
Potential Celiac Disease Vaccines
A potential celiac disease vaccine could one day be a reality. Whenever new information is available we update our Research News and our Celiac Disease Vaccine page .
Celiac Disease Clinical Trials
Researchers around the world are working to develop new treatments for celiac disease. As a person affected by celiac disease, you can play an important role in advancing research by participating in clinical trials . View our Clinical Trials Infographic to learn how clinical trials tie into to drug development process.
Additionally, Beyond Celiac has been directly involved with assisting a number of clinical studies in all phases. With the largest celiac disease social community, a robust email and research database and a website that receives over 2 million visits a year, we are uniquely qualified to help your clinical trial recruit qualified candidates. Learn about our patient recruitment offerings .
Celiac Disease Research Symposiums & Summits
2019 Symposium
2019 Summit
2018 Symposium
2015 Summit
2022 Summit
Meet Our Science Team
Our Chief Scientific Officers lead the development and implementation of a transformational patient-centered research agenda focused on accelerating solutions toward new pharmaceutical treatments and a cure for celiac disease.
Our Chief Scientist and Strategy Officer develops our research and funding priorities to impact patients in areas including clinical decision-making about disease management, treatment, and eventually a cure.
Learn more about our Science Team , as well as our Medical Advisory Council and Scientific Advisory Board .
Think you may have celiac disease?
Opt-in to stay up-to-date on the latest news.
SLAC and Stanford researchers advance understanding of a key celiac enzyme
Wheat and other sources of gluten can spell trouble for people with the disease, but new findings could aid the development of first-ever drugs for the autoimmune disorder.
By Nathan Collins

Celiac disease affects around one in a hundred people worldwide , and those that have the autoimmune disorder have no choice but to stick to a gluten-free diet forever – at the moment, doctors have no other way to treat the illness.
Now, those seeking a treatment could get a boost: A new study from researchers at Stanford University and the Stanford Synchrotron Radiation Lightsource (SSRL) at the U.S. Department of Energy's SLAC National Accelerator Laboratory has revealed previously unseen details of a key enzyme behind the disease. The study was published in Proceedings of the National Academy of Sciences .
Celiac scientists have known for several years now that the enzyme in question, transglutiminase 2 (TG2), could trigger an immune response in the presence of gluten and calcium ions, leading the body to attack its own intestinal tissues. What has been less clear is exactly how TG2 works and how best to target it with drugs — in part because scientists have only had a limited understanding of the enzyme's structure. Previous studies have mapped out TG2's inactive, "closed" state and its active, "open" state, but how it transformed from one to the other or what happens in the interim remained unclear.
To address that problem, Angele Sewa, a graduate student in chemistry and biochemistry in Stanford chemist Chaitan Khosla's lab and a fellow in the Sarafan ChEM-H Chemistry/Biology Interface Training Program , and Harrison Besser, a student in Stanford's Medical Scientist Training Program , first worked to create complexes of TG2, calcium ions and gluten-like substances that could reveal in greater detail how TG2 works. Sewa next grew crystals of those complexes and worked with SSRL lead scientist Irimpan Mathews to understand their structures using X-ray macromolecular crystallography.
The effort was a success: Several of the crystallization concoctions the team came up with captured TG2 in a previously unobserved intermediate state between its active and inactive states. Analyzing the structure of this intermediate state, the researchers wrote, uncovered a wealth of details about how TG2 interacts with gluten and calcium, helping to make sense of previous results about TG2's behavior as well as identifying specific sites within TG2 that play key roles in its activity.
While researchers are already developing drugs targeting TG2 for celiac disease and another TG2-related disease, idiopathic pulmonary fibrosis, "this study provides fundamentally new structural and mechanistic insight into how TG2-inhibiting drugs work," Khosla said. "These insights can be used to make better drugs against this target."
SSRL is a DOE Office of Science user facility. The SSRL Structural Molecular Biology program is supported by the DOE Office of Science and the NIH National Institute of General Medical Sciences.
Citation: Agnele S. Sewa, et al., Proceedings of the National Academy of Sciences, 3 July 2024 ( 10.1073/pnas.2407066121 )
For questions or comments, contact SLAC Strategic Communications & External Affairs at [email protected] .
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science . The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.
Related Topics
- Science news
- Biological sciences
- Structural molecular biology
- X-ray crystallography
- X-ray science
- X-ray light sources and electron imaging
- Stanford Synchrotron Radiation Lightsource (SSRL)
Related stories
A novel spray device helps researchers capture fast-moving cell processes.
Researchers figured out how to spray and freeze a cell sample in its natural state in milliseconds, helping them capture basic biological processes in...

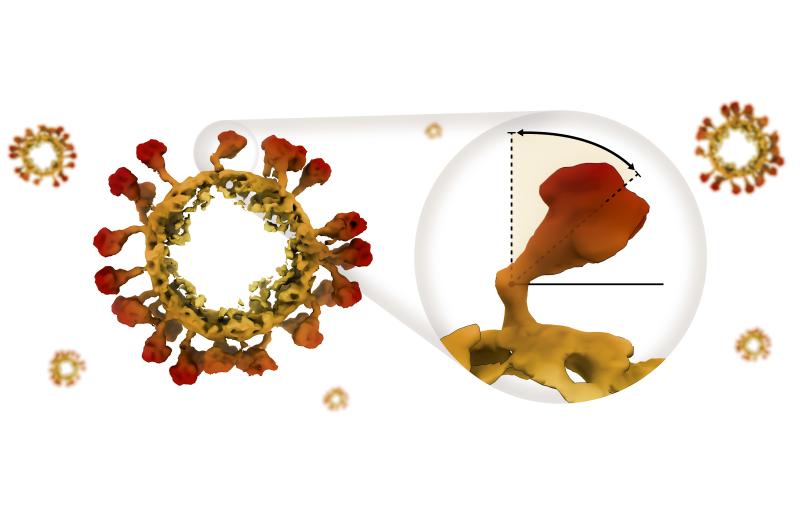
How tiny hinges bend the infection-spreading spikes of a coronavirus
Disabling those hinges could be a good strategy for designing vaccines and treatments against a broad range of coronavirus infections.
Illuminating the dance of RNA with ultrabright X-rays
Scientists developed a new method to unlock the secrets of RNA. The implications are wide-reaching, from better understanding diseases to designing new therapeutics.
Tearing apart a million-dollar microscope – for science
Peter Dahlberg has combined two complex imaging techniques into one. The 2021 Panofsky Fellow adds cryo-ET and biosensors to fluorescence microscopy to give context...
SLAC fires up the world’s most powerful X-ray laser: LCLS-II ushers in a new era of science
With up to a million X-ray flashes per second, 8,000 times more than its predecessor, it transforms the ability of scientists to explore atomic-scale...

Researchers find new molecule that shows promise in slowing SARS-CoV-2
A molecule with hooks that can grip and disable the virus’s pesky protease shows potential for fighting infection.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 July 2024
A human autoimmune organoid model reveals IL-7 function in coeliac disease
- António J. M. Santos 1 ,
- Vincent van Unen ORCID: orcid.org/0000-0001-9339-8430 1 , 2 , 3 ,
- Zhongqi Lin ORCID: orcid.org/0000-0002-4806-0091 1 ,
- Steven M. Chirieleison ORCID: orcid.org/0000-0002-3997-5652 1 , 4 ,
- Nhi Ha ORCID: orcid.org/0000-0001-5782-0921 1 ,
- Arpit Batish 1 ,
- Joshua E. Chan ORCID: orcid.org/0000-0002-6016-1397 1 ,
- Jose Cedano 1 ,
- Elisa T. Zhang 1 ,
- Qinghui Mu 1 ,
- Alexander Guh-Siesel 1 ,
- Madeline Tomaske 1 ,
- Deana Colburg 4 ,
- Sushama Varma 4 ,
- Shannon S. Choi 1 ,
- Asbjørn Christophersen 5 , 6 , 7 ,
- Ani Baghdasaryan 8 ,
- Kathryn E. Yost ORCID: orcid.org/0000-0001-6807-950X 9 , 10 ,
- Kasper Karlsson 1 , 11 , 12 ,
- Andrew Ha 1 ,
- Jing Li 3 ,
- Hongjie Dai 8 ,
- Zachary M. Sellers ORCID: orcid.org/0000-0001-5352-7760 13 ,
- Howard Y. Chang ORCID: orcid.org/0000-0002-9459-4393 9 , 10 , 12 , 14 ,
- James C. Y. Dunn ORCID: orcid.org/0000-0002-6826-7065 15 ,
- Bing M. Zhang 4 ,
- Elizabeth D. Mellins ORCID: orcid.org/0000-0003-2577-139X 13 ,
- Ludvig M. Sollid ORCID: orcid.org/0000-0001-8860-704X 5 , 6 ,
- Nielsen Q. Fernandez-Becker 16 ,
- Mark M. Davis ORCID: orcid.org/0000-0001-6868-657X 2 , 3 , 14 &
- Calvin J. Kuo ORCID: orcid.org/0000-0002-7427-5985 1
Nature ( 2024 ) Cite this article
5326 Accesses
59 Altmetric
Metrics details
- Autoimmunity
- Immunopathogenesis
In vitro models of autoimmunity are constrained by an inability to culture affected epithelium alongside the complex tissue-resident immune microenvironment. Coeliac disease (CeD) is an autoimmune disease in which dietary gluten-derived peptides bind to the major histocompatibility complex (MHC) class II human leukocyte antigen molecules (HLA)-DQ2 or HLA-DQ8 to initiate immune-mediated duodenal mucosal injury 1 , 2 , 3 , 4 . Here, we generated air–liquid interface (ALI) duodenal organoids from intact fragments of endoscopic biopsies that preserve epithelium alongside native mesenchyme and tissue-resident immune cells as a unit without requiring reconstitution. The immune diversity of ALI organoids spanned T cells, B and plasma cells, natural killer (NK) cells and myeloid cells, with extensive T-cell and B-cell receptor repertoires. HLA-DQ2.5-restricted gluten peptides selectively instigated epithelial destruction in HLA-DQ2.5-expressing organoids derived from CeD patients, and this was antagonized by blocking MHC-II or NKG2C/D. Gluten epitopes stimulated a CeD organoid immune network response in lymphoid and myeloid subsets alongside anti-transglutaminase 2 (TG2) autoantibody production. Functional studies in CeD organoids revealed that interleukin-7 (IL-7) is a gluten-inducible pathogenic modulator that regulates CD8 + T-cell NKG2C/D expression and is necessary and sufficient for epithelial destruction. Furthermore, endogenous IL-7 was markedly upregulated in patient biopsies from active CeD compared with remission disease from gluten-free diets, predominantly in lamina propria mesenchyme. By preserving the epithelium alongside diverse immune populations, this human in vitro CeD model recapitulates gluten-dependent pathology, enables mechanistic investigation and establishes a proof of principle for the organoid modelling of autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease
mTOR sustains inflammatory response in celiac disease
Tolerance-inducing therapies in coeliac disease — mechanisms, progress and future directions
Data availability.
Data sets for scRNA-seq have been deposited in Gene Expression Omnibus with the accession code GSE200075 . Source data are provided with this paper.
Catassi, C., Verdu, E. F., Bai, J. C. & Lionetti, E. Coeliac disease. Lancet 399 , 2413–2426 (2022).
Article PubMed Google Scholar
Levescot, A., Malamut, G. & Cerf-Bensussan, N. Immunopathogenesis and environmental triggers in coeliac disease. Gut 71 , 2337–2349 (2022).
Article CAS PubMed Google Scholar
Iversen, R. & Sollid, L. M. The immunobiology and pathogenesis of celiac disease. Annu. Rev. Pathol. 18 , 47–70 (2023).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102 , 330–354 (1992).
Jabri, B. et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 118 , 867–879 (2000).
Hüe, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21 , 367–377 (2004).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21 , 357–366 (2004).
Pinto-Sanchez, M. I. et al. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18 , 875–884 (2021).
Article PubMed PubMed Central Google Scholar
Barone, M. V. et al. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS ONE 6 , e17039 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar
Castellanos-Rubio, A. et al. Long-term and acute effects of gliadin on small intestine of patients on potentially pathogenic networks in celiac disease. Autoimmunity 43 , 131–139 (2010).
Palova-Jelinkova, L. et al. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J. Immunol. 175 , 7038–7045 (2005).
Freire, R. et al. Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease. Sci. Rep. 9 , 7029 (2019).
Article ADS PubMed PubMed Central Google Scholar
de Kauwe, A. L. et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4 + T cells. J. Immunol. 182 , 7440–7450 (2009).
Abadie, V. et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 578 , 600–604 (2020).
Goel, G. et al. Serum cytokines elevated during gluten-mediated cytokine release in coeliac disease. Clin. Exp. Immunol. 199 , 68–78 (2020).
Tye-Din, J. A. et al. Patient factors influencing acute gluten reactions and cytokine release in treated coeliac disease. BMC Med. 18 , 362 (2020).
Article CAS PubMed PubMed Central Google Scholar
Jabri, B. & Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 15 , 771–783 (2015).
Lähdeaho, M.-L. et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol. 4 , 948–959 (2019).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γ c family cytokines. Nat. Rev. Immunol. 9 , 480–490 (2009).
Lei, X. et al. Down-regulation of interleukin 7 receptor (IL-7R) contributes to central nervous system demyelination. Oncotarget 8 , 28395–28407 (2017).
Churchman, S. M. et al. Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis. Arthritis Res. Ther. 16 , 511 (2014).
Penaranda, C. et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc. Natl Acad. Sci. USA 109 , 12668–12673 (2012).
Lee, L. -F. et al. Anti-IL-7 receptor-α reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl Acad. Sci. USA 109 , 12674–12679 (2012).
Belarif, L. et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J. Clin. Invest. 129 , 1910–1925 (2019).
Watanabe, M. et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J. Exp. Med. 187 , 389–402 (1998).
Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572 , 481–487 (2019).
Li, J. et al. KIR + CD8 + T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376 , eabi9591 (2022).
Sollid, L. M. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics 69 , 605–616 (2017).
Anderson, R. P., Degano, P., Godkin, A. J., Jewell, D. P. & Hill, A. V. S. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6 , 337–342 (2000).
Petersen, J. et al. T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat. Struct. Mol. Biol. 21 , 480–488 (2014).
Sollid, L. M. et al. Update 2020: nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4 + T cells. Immunogenetics 72 , 85–88 (2020).
Sette, A., Southwood, S., Miller, J. & Appella, E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J. Exp. Med. 181 , 677–683 (1995).
Korneychuk, N. et al. Interleukin 15 and CD4 + T cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology 146 , 1017–1027 (2014).
Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547 , 94–98 (2017).
Broughton, S. E. et al. Biased T cell receptor usage directed against human leukocyte antigen DQ8-restricted gliadin peptides is associated with celiac disease. Immunity 37 , 611–621 (2012).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38 , 1194–1202 (2020).
Dahal-Koirala, S. et al. Comprehensive analysis of CDR3 sequences in gluten-specific T-cell receptors reveals a dominant R-motif and several new minor motifs. Front. Immunol. 12 , 639672 (2021).
Galeano Niño, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife 9 , e56554 (2020).
Christophersen, A., Risnes, L. F., Dahal-Koirala, S. & Sollid, L. M. Therapeutic and diagnostic implications of T cell scarring in celiac disease and beyond. Trends Mol. Med. 25 , 836–852 (2019).
Hamilton, J. A. GM-CSF-dependent inflammatory pathways. Front. Immunol. 10 , 2055 (2019).
Olaussen, R. W. et al. Interferon-γ-secreting T cells localize to the epithelium in coeliac disease. Scand. J. Immunol. 56 , 652–664 (2002).
Husby, S. et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 70 , 141–156 (2020).
Di Niro, R. et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 18 , 441–445 (2012).
Kivelä, L. et al. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18 , 181–195 (2021).
Dooms, H. Interleukin-7: fuel for the autoimmune attack. J. Autoimmun. 45 , 40–48 (2013).
Meresse, B. et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 203 , 1343–1355 (2006).
Lundin, K. E. et al. Gliadin-specific, HLA-DQ(α1*0501,β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178 , 187–196 (1993).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15 , 701–706 (2009).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175 , 1972–1988 (2018).
Maiuri, L. et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362 , 30–37 (2003).
Yang, H., Spencer, A. U. & Teitelbaum, D. H. Interleukin-7 administration alters intestinal intraepithelial lymphocyte phenotype and function in vivo. Cytokine 31 , 419–428 (2005).
Porter, B. O. & Malek, T. R. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the γc-dependent cytokines IL-2, IL-7, and IL-15. Semin. Immunol. 12 , 465–474 (2000).
Dong, L.-H., Lv, P. & Han, M. Roles of SM22α in cellular plasticity and vascular diseases. Cardiovasc. Hematol. Disord. Drug Targets 12 , 119–125 (2012).
Watanabe, M. et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95 , 2945–2953 (1995).
Christophersen, A. et al. Distinct phenotype of CD4 + T cells driving celiac disease identified in multiple autoimmune conditions. Nat. Med. 25 , 734–737 (2019).
Gandini, A., Gededzha, M. P., De Maayer, T., Barrow, P. & Mayne, E. Diagnosing coeliac disease: a literature review. Hum. Immunol. 82 , 930–936 (2021).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16 , 1226–1232 (2019).
Stirling, D. R. et al. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 22 , 433 (2021).
Ráki, M. et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl Acad. Sci. USA 104 , 2831–2836 (2007).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184 , 3573–3587 (2021).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15 , 1484–1506 (2020).
Download references
Acknowledgements
We thank members of the Kuo, Davis, Mellins and Sollid groups for discussions; the Stanford FACS, Functional Genomics, Human Histology (P. Chu), Cell Sciences Imaging and Human Immune Monitoring cores for technical expertise; the Stanford Tissue Bank for providing surgical samples; E. Sanjines, A. Adiao, D. Souki, G. Tan and G. Masarweh for collection and delivery of endoscopy samples; B. Simonsen for the HLA-DQ molecules used for tetramer assembly; and J. and R. Triebsch for support from the Stanford Celiac Translational Research Program. This work was also supported by funding from the Stanford Medicine Children’s Health Center for IBD and Celiac Disease. V.v.U. was supported by a Netherlands Organization for Scientific Research Rubicon grant (452181214). We acknowledge funding from the South-Eastern Norway Regional Health Authority (projects 2016113 and 2020027 to L.M.S.), a Stanford Maternal Child Health Research Institute seed grant (C.J.K.), NIH RM1-HG007735 (H.Y.C.), NIH U19AI057229 (M.M.D.), NIH U01DK085527, U19AI116484, R01CA251514, R01DK130414, R01DK115728 (C.J.K.), the NIDDK Intestinal Stem Cell Consortium and the NIAID Biomimetic U19 Consortium. We dedicate this work to the memory of Elizabeth D. Mellins, whose experimental design and guidance were crucial for this study.
Author information
Authors and affiliations.
Division of Hematology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
António J. M. Santos, Vincent van Unen, Zhongqi Lin, Steven M. Chirieleison, Nhi Ha, Arpit Batish, Joshua E. Chan, Jose Cedano, Elisa T. Zhang, Qinghui Mu, Alexander Guh-Siesel, Madeline Tomaske, Shannon S. Choi, Kasper Karlsson, Andrew Ha & Calvin J. Kuo
Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA
Vincent van Unen & Mark M. Davis
Institute for Immunity, Transplantation and Infection, Stanford University School of Medicine, Stanford, CA, USA
Vincent van Unen, Jing Li & Mark M. Davis
Department of Pathology, Stanford University School of Medicine, Stanford, CA, USA
Steven M. Chirieleison, Deana Colburg, Sushama Varma & Bing M. Zhang
K. G. Jebsen Coeliac Disease Research Centre, Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Asbjørn Christophersen & Ludvig M. Sollid
Department of Immunology, Oslo University Hospital, Oslo, Norway
Department of Rheumatology, Dermatology and Infectious Diseases, Oslo University Hospital, Oslo, Norway
Asbjørn Christophersen
Department of Chemistry, Stanford University School of Medicine, Stanford, CA, USA
Ani Baghdasaryan & Hongjie Dai
Center for Personal Dynamic Regulomes, Stanford University School of Medicine, Stanford, CA, USA
Kathryn E. Yost & Howard Y. Chang
Department of Dermatology, Stanford University School of Medicine, Stanford, CA, USA
Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
Kasper Karlsson
Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA
Kasper Karlsson & Howard Y. Chang
Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA
Zachary M. Sellers & Elizabeth D. Mellins
Howard Hughes Medical Institute, Stanford University School of Medicine, Stanford, CA, USA
Howard Y. Chang & Mark M. Davis
Department of Pediatric Surgery, Stanford University School of Medicine, Stanford, CA, USA
James C. Y. Dunn
Division of Gastroenterology and Hepatology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
Nielsen Q. Fernandez-Becker
You can also search for this author in PubMed Google Scholar
Contributions
A.J.M.S. conceived, designed and performed experiments, analysed data and wrote the manuscript. V.v.U. conceived experiments and analysed scRNA-seq data. Z.L. designed experiments, did organoid cultures, confocal imaging and analysis, analysed scRNA-seq data and did RT–qPCR. S.M.C. collected tissue FFPE blocks and coordinated sectioning for haplotyping and IL7 in situ hybridization and analysed data. N.H. did organoid cultures and RT–qPCR. A. Batish did RT–qPCR. J.E.C. measured and analysed organoid sizes. J.C. did organoid cultures, cryopreservation and recovery, imaging and analysis. E.T.Z. sectioned frozen tissue blocks and helped with their staining and imaging. Q.M. did organoid cultures. A.G.-S. did confocal imaging and analysis. M.T. did RT–qPCR and data analysis. D.C. sectioned FFPE tissue blocks and did IL7 in situ hybridization. S.V. did IL7 in situ hybridization. S.S.C. did confocal imaging and schematic design. A.C. produced HLA-DQ monomers for tetramer assembly. A. Baghdasaryan provided resources. K.E.Y. prepared libraries for scRNA-seq. K.K. used Cell Ranger for scRNA-seq. A.H. assisted with 10x Genomics cell capture. J.L. did HLA genotyping. H.D. provided resources and supervision. Z.M.S. provided samples and guidance. H.Y.C. provided resources and supervision. J.C.Y.D. provided surgical samples. B.M.Z. did HLA genotyping and analysis. E.D.M. conceived and designed experiments and provided resources and supervision. L.M.S. provided resources, supervision and guidance. N.Q.F.-B. identified CeD patients and controls, coordinated and collected endoscopy samples and provided guidance. M.M.D. provided resources, supervision and guidance. C.J.K. conceived and designed experiments, analysed data and wrote the manuscript.
Corresponding author
Correspondence to Calvin J. Kuo .
Ethics declarations
Competing interests.
C.J.K. and A.J.M.S. are inventors on patent WO 2020/247528 describing methods and uses of patient-derived celiac intestinal organoids. C.J.K. and M.M.D. are founders of Mozart Therapeutics and NextVivo, Inc. L.M.S has been a consultant during the last 3 years for BMS, GSK, Mozart Therapeutics, Ono Pharmaceutical, Precigen ActoBio, Sanofi-Aventis, SQZ Biotech, Takeda and Topas Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information.
Nature thanks Nadine Cerf-Bensussan, Toshiro Sato and Detlef Schuppan for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended data fig. 1 small intestine ali organoids possess different mesenchymal and epithelial cell types..
a , IF whole-mount staining of small intestine organoids at day 14 showing SMA + or PDGFRA + fibroblasts, CD31 + endothelial cells and PGP9.5 + neurons (green), ECAD + epithelium (white) and DAPI (blue) (representative images from n = 3 biological replicates). b , IF whole-mount staining of small intestine organoids at day 14 showing MUC2 + goblet cells, CHGA + enteroendocrine cells, LYZ1 + Paneth cells (green), ECAD + epithelium (white) and DAPI (blue) (representative images from n = 3 biological replicates). c , Enlargement of organoid cup-shaped MUC2 + goblet cells (representative image from n = 3 biological replicates). d , Violin plots of CD14 and CD68 mRNA expression from CeD organoid scRNA-seq and a scatter plot of CD14 and CD68 mRNA co-expression in the myeloid compartment. e , Whole-mount IF staining of small intestine ALI organoid CD4 + (green) and CD8 + (red) T cells, showing enrichment of CD8 + T cells within the EPCAM + epithelial compartment (white). DAPI (blue). In contrast, CD4 + T cells localize to non-epithelial lamina propria-like areas (representative image from n = 3 biological replicates). All scale bars are 100 µm, except (c) in which the scale bar is 50 µm.
Extended Data Fig. 2 Duodenal ALI organoids contain diverse immune populations, related to Fig. 1 .
a , Integrated UMAP plot of CD45 + -sorted cells from scRNA-seq, revealing diverse immune populations in small intestine ALI organoids at day 14, n = 6 CeD patients. b , Violin plots showing expression of genes used to identify the immune populations shown in (a). c , UMAP plots of overlap between tissue and organoid CD45 + immune populations as in (a, b). d , scRNA-seq Jaccard index of TCR overlap between fresh small intestine tissue ( n = 1 CeD patient) and ALI organoids ( n = 4 CeD patients). e , Integrated UMAP from scRNA-seq of active CeD organoid T cells ( n = 6 patients). Cells expressing KIR3DL1 or KIR2DL3 are rendered in red. f , Plot of CD8 + T cells from (e). g , Integrated UMAP from scRNA-seq of active CeD organoid T cells (top left) ( n = 6 patients). Cells in red exhibit expression of KIR3DL1 or KIR2DL3 (top right), NKG2C (bottom left) and NKG2D (bottom right). h , Pie bar graph showing organoid-derived TCR counts in which each segment represents a unique clonotype, n = 5 patients. Expanded clonotypes (TCR counts ≥ 2) are indicated in red.
Extended Data Fig. 3 Cytokine supplementation and cryopreservation of intestinal ALI organoids.
a , FACS-based tSNE plots depicting time course abundance of EPCAM + and CD45 + cells (top) and CD4 + and CD8 + T cells (bottom) as a percentage of total live single ileal organoid cells with or without addition of IL-2 and IL-7, representative experiment of n = 3 biological replicates. b , Organoids grown for 14 days (control) have similar percentages of epithelium and immune components as organoids grown for 5 days, frozen in-gel at −80 °C, cryorecovered, and replated for the indicated durations. c-d , ALI organoids demonstrate persistent growth after being frozen in-gel at −80 °C, cryorecovered and replated (c, arrows), with maintenance of epithelial protrusions by H&E (d). Numerous air bubbles in the collagen are present on initial plating post-cryorecovery and progressively disappear with culture. (b-d) depict representative experiments from n = 4 biological replicates. Scale bar is 5 mm for (c) and 100 µm for (d).
Extended Data Fig. 4 Gliadin induces loss of villus-like structures in CeD organoids.
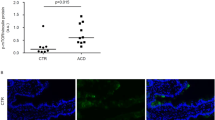
a , Duodenal ALI organoids from celiac (CeD) or non-celiac control donors were established for 9–12 days followed by gliadin or CLIP treatment for 2 days before analysis, unless stated otherwise. The gliadin peptides were a 1:1 mixture of deamidated immunodominant, HLA-DQ2.5-restricted, glia-α1 (LQPFPQPELPYPGS) and glia-α2 (APQPELPYPQPGS) gluten epitopes. b - d , Confirmatory IF staining of sections of human duodenum tissue showing IL-15 (red) in (a), SI (red) in (b) and APOA4 (red) in (c); DAPI (blue) (representative images from n = 3 biological replicates). e , Quantification of SI mRNA in FACS-sorted organoid EPCAM + cells from 2-day gliadin-treated control or active CeD organoids. RT-qPCR, expressed as a ratio of gliadin:CLIP treatment, from control ( n = 4) or CeD ( n = 5) biological replicates. Box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. *, P = 0.0381; two-tailed Mann-Whitney test. f , Representative H&E staining of different sections of control or active CeD organoids after 2-day gliadin or CLIP treatment. Arrows denote regions where epithelial protrusions are absent. g , Quantification of epithelial protrusions per organoid circumference from (f); control (N = 6 biological replicates), CeD (N = 7 biological replicates), each data point is from an individual organoid. Scatter plots show the median as the center line and the whiskers represent the min and max. ***= P < 0.0001; two-tailed Mann-Whitney test. All scale bars are 100 µm. All CeD organoids were DQ2.5 + .
Source Data
Extended Data Fig. 5 Gliadin induces epithelial proliferation in CeD organoids.
a , Representative IF staining of sections of active CeD organoids after 2-day gliadin or CLIP treatment in EN media showing proliferative KI67 + cells (green), ECAD (red) and DAPI (blue). Scale bar is 50 µm. b , Quantification of KI67 fluorescence from (a), control ( n = 3 biological replicates), CeD ( n = 5 biological replicates); each data point is from an individual organoid. ***, P < 0.0001; two-tailed Mann-Whitney test. c , Representative brightfield images of active CeD organoids before and after 2-day treatment with gliadin or CLIP peptides. Scale bar is 5 mm. d , Automated quantification of fold change in CeD organoid area from (c), 2 days after treatment with gliadin or CLIP. n = 10 CeD patients. **, P = 0.002; two-tailed Wilcoxon test. e , LGR5 RT-qPCR from FACS-sorted organoid EPCAM + cells as ratio of gliadin:CLIP treatment for 2 days in organoids from control ( n = 7 biological replicates) or active CeD ( n = 8 biological replicates). **, P = 0.0012; two-tailed Mann-Whitney test. f , PCNA RT-qPCR from FACS-sorted organoid EPCAM + cells as ratio of gliadin:CLIP treatment for 2 days in control or active CeD organoids, ( n = 7 biological replicates each). **, P = 0.007; two-tailed Mann-Whitney test. g , CCND1 RT-qPCR from FACS-sorted organoid EPCAM + cells as ratio of gliadin:CLIP treatment for 2 days in organoids from control ( n = 8 biological replicates) or active CeD ( n = 7 biological replicates). ***, P = 0.0003; two-tailed Mann-Whitney test. All box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. All CeD organoids were DQ2.5 + .
Extended Data Fig. 6 TCR sequencing from CeD organoid scRNA-seq reveals known and suspected gliadin-specific TCR motifs.
a , scRNA-seq integrated UMAP plot highlighting TCR-expressing T cells in active CeD organoids at day 14, n = 5 CeD patients. b , GLIPH homology analysis showing conserved CDR3 motifs (red) found between active CeD organoids and gliadin-specific published sequences, n = 5 CeD patients. All CeD organoids in this figure were HLA-DQ2.5 + .
Extended Data Fig. 7 Two additional biological replicates of scRNA-seq-derived dot plots.
Dot plots from organoid scRNA-seq from two active CeD patients (a, b); a third patient is shown in Fig. 4a . Depiction of mean expression levels and corresponding percent population expression amongst active CeD organoid CD4 + and CD8 + T cells, Treg, plasma B cells and myeloid cells after 2-day gliadin or CLIP treatment. The patient in (a) is HLA-DQ2.5, as is the patient in Fig. 4a . The patient in (b) is HLA-DQ2.2, which manifests low-affinity binding to the HLA-DQ2.5 gliadin peptides used in the study (Bodd et al, Gastroenterology, 2012 Mar;142(3):552-61).
Extended Data Fig. 8 ScRNA-seq-based interactome analysis of novel gliadin-induced immune interactions in CeD organoids.
a , Overview of unique CellPhoneDB immune interactions found in 2-day CLIP- or gliadin-treated active CeD organoids, stratified by immune cell type (CD4 + T, CD8 + T, myeloid, NK and Treg cells). Columns indicate sending:receiving cell type and rows indicate ligand-receptor pairs. P values are indicated by circle size. The mean (log 2 ) average expression levels of interacting molecule 1 and interacting molecule 2 are indicated by the color gradient. b , Corresponding schematic showing potential interactions between immune cells in CeD. Integrated data from n = 4 CeD patients, 3 DQ2.5 + and 1 DQ2.2 + .
Extended Data Fig. 9 Sc-RNAseq-based interactome of B cells and plasma cells, and BCR sequence consensus.
a , B cell- and plasma B cell-specific immune interactomes derived from scRNA-seq CellPhoneDB analysis showing 117 unique B and plasma-cell driven interactions in gliadin-treated organoids and absence of unique interactions in CLIP, integrated data from n = 4 CeD patients. b , BCR sequence consensus analysis from scRNA-seq of matched CDR3 sequences from active CeD organoids and published anti-TG2 CDR3 sequences categorized by length; n = 3 CeD patients.
Extended Data Fig. 10 BCR sequencing from CeD organoid scRNA-seq reveals extensive overlap with public anti-TG2 CeD-specific motifs.
a , scRNA-seq integrated UMAP plot highlighting BCR-expressing B and plasma cells in active CeD organoids, n = 3 CeD patients. b , Homology analysis showing conserved CDR3 sequences (red) found between active CeD organoids and anti-TG2 CeD-specific published sequences, n = 3 CeD patients.
Extended Data Fig. 11 IL-7 is upregulated in active celiac duodenal biopsy tissue.
a , Luminex protein analysis of organoid conditioned media from active CeD (N = 7 biological replicates) or control (N = 4 biological replicates) showing fold-increases of IL-7 as ratio of gliadin:CLIP treatment after 2 days. Box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. ns, P = 0.072; two-tailed Mann-Whitney test. b - c , Representative IF staining using a rabbit (Rb) anti-IL-7 antibody (red) in fresh duodenal biopsies from (b) 14 remission CeD patients (previously diagnosed with CeD but on gluten-free diet) versus (c) 14 CeD patients with active disease, showing increased IL-7 levels in the latter. Epithelium (CK19, green); DAPI (blue). Figure 6c shows staining for a 15 th patient in remission and a 15 th patient with active CeD, and quantitation is presented in Fig. 6d . (GFD, n = 15 donors) or active CeD ( n = 15 donors). d , Representative IF staining using a mouse (Ms) anti-IL-7 antibody (red) in fresh duodenal biopsies from (b), 4 remission CeD patients versus 4 patients with active CeD. This confirmed elevated IL-7 expression in active CeD seen with a different antibody than in (b) and (c). Scale bars are 100 µm.
Supplementary information
Supplementary information.
This file contains sequential FACS gating strategy, donor DQ-typing and demographics, and primer sequences used for RT-qPCR.
Reporting Summary
Source data, source data figs. 2–6 and source data extended data figs. 4, 5, 10., rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Santos, A.J.M., van Unen, V., Lin, Z. et al. A human autoimmune organoid model reveals IL-7 function in coeliac disease. Nature (2024). https://doi.org/10.1038/s41586-024-07716-2
Download citation
Received : 04 April 2022
Accepted : 14 June 2024
Published : 24 July 2024
DOI : https://doi.org/10.1038/s41586-024-07716-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

New Insights into Non-Dietary Treatment in Celiac Disease: Emerging Therapeutic Options
To date, the only treatment for celiac disease (CD) consists of a strict lifelong gluten-free diet (GFD), which has numerous limitations in patients with CD. For this reason, dietary transgressions are frequent, implying intestinal damage and possible long-term complications. There is an unquestionable need for non-dietary alternatives to avoid damage by involuntary contamination or voluntary dietary transgressions. In recent years, different therapies and treatments for CD have been developed and studied based on the degradation of gluten in the intestinal lumen, regulation of the immune response, modulation of intestinal permeability, and induction of immunological tolerance. In this review, therapeutic lines for CD are evaluated with special emphasis on phase III and II clinical trials, some of which have promising results.
1. Introduction
Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by exposure to dietary gluten in genetically predisposed individuals [ 1 , 2 ]. The pooled global prevalence of CD has been reported to be approximately 1%, however, the prevalence values for CD varies in South America, Africa, North America, Asia, Europe, and Oceania; the prevalence is higher in female vs. male individuals and is 4–8 times higher among non-Hispanic white people compared with other races. Moreover, there has been an increase in the diagnosis rate in the last 10 years [ 3 , 4 , 5 , 6 , 7 ]. CD is characterized by intestinal and/or extraintestinal manifestations, elevation of specific antibodies such as anti-gliadin and anti-tissue transglutaminase (anti-tTG), and the presence of HLA-DQ2/DQ8 haplotypes [ 8 , 9 , 10 , 11 ].
Gluten is a complex mixture of seed storage proteins known as prolamins, found in cereals grains such as wheat, barley, rye, oats, and their derivatives. The viscoelastic network generated by gluten enables an excellent aerated structure, contributing to the baking quality of these cereals [ 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ]. Gluten proteins are characterized by high proline and glutamine content. Therefore, these proteins are partially degraded to peptides by digestive proteases of the gastrointestinal track that persist in the intestine and potentiate their deamidation through tTG [ 13 ].
The prevailing hypothesis of immunopathogenesis is the two-signal model, which establishes that gluten has a dual effect on the duodenum of celiac patients mediated by innate and adaptive immune systems [ 14 , 15 ]. Certain peptides, such as the 19-mer gliadin peptide, trigger an innate immune response mainly characterized by the production of interleukin-15 (IL-15) by epithelial cells and the disruption of the epithelial barrier caused by increased permeability and induction of enterocyte apoptosis [ 16 , 17 ]. Consequently, other peptides such as the 33-mer gliadin can now reach the lamina propria to be deamidated by tTG, providing a negative charge to gliadin peptides that activate the immune-adaptive system. The affinity of the HLA-DQ2/8 peptide is enhanced and expressed on the surface of dendritic cells (DCs) [ 18 , 19 , 20 ]. DCs present a gluten antigen to T-cells and drive the progression of the proinflammatory response, thereby contributing to the symptomatology of the disease [ 21 , 22 ].
2. Gluten-Free Diet: Challenge and Gluten Exposure
Currently, the only available treatment for CD is a strict, lifelong gluten-free diet (GFD). Dietary gluten restriction is a safe and effective therapy; however, unintentional gluten exposure on a GFD is common and intermittent. Recent findings suggest that most CD patients can only attain a gluten-reduced diet instead of the recommended strict GFD. Gluten exposure may be more common than realized and is distinct from lapses in an otherwise intentionally strict GFD [ 23 , 24 ].
Among the main causes of gluten exposure in a GFD is the ubiquitous nature of gluten, food cross-contamination, and the limitations and socio-emotional toll [ 25 ]. In addition, many of the manufactured gluten-free products tend to be less healthy than their gluten analogues since high amounts of lipids, sugars, and other additives are incorporated in their production to simulate the viscoelastic properties of gluten proteins [ 26 ]. Although it is well known that legislation on the labeling of gluten-free products is based on the limitation of 20 parts per million (ppm) of gluten [ 27 ], there is no clear consensus on the safe amount of daily gluten intake due to the threshold for triggering symptoms has interindividual variability. Total daily gluten consumption that seems to be safe for most CD patients is <50 mg gluten; nevertheless, little amounts as 10 mg of daily gluten for some CD patients could promote development of intestinal mucosal abnormalities [ 28 ].
Several studies based on nutritional questionnaires, serological tests, and evaluating gluten immunogenic peptides in feces and urine, have reported variable gluten exposure rates in patients with CD, reaching up to 69% in adults, 64% in adolescents, and 45% in children ( Figure 1 ) despite their best efforts to avoid it. Studies reporting gluten exposure rates may compromise high rates of ongoing symptoms [ 29 , 30 , 31 ] and enteropathy [ 32 , 33 , 34 , 35 ] in patients with CD, leading to comorbidities such as anemia, severe malabsorption, and various forms of malignancies [ 36 ]. Hence, it is important to drive efforts to develop non-dietary adjunctive or alternative therapies for CD treatment [ 37 ]. Recently, researchers have attempted to meet the requests of celiac patients seeking therapies aside from GFD. In this review, we summarize the spectrum of potential therapeutic agents to improve CD management and their research status, highlighting several drug candidates in phase II/III clinical trials.

Studies reporting gluten exposure rates in CD patients on a supposed GFD. CD, celiac disease; GFD, gluten-free diet [ 23 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ].
3. Potential Alternative or Adjuvant Non-Dietary Treatments for CD
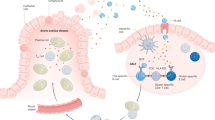
The emerging therapeutic options for CD can be broadly classified into one of the following strategies—(1) removal of toxic gluten peptides before reaching the intestine, (2) regulation of the immunostimulatory effects of toxic gluten peptides, (3) modulation of intestinal permeability, (4) immune modulation and induction of gluten tolerance, and (5) restoration of the imbalance in the gut microbiota ( Figure 2 ).

Emerging therapeutic approaches for non-dietary CD treatment. APC, antigen-presenting cell; CD, celiac disease; IL-15, interleukin 15; TNF, tumor necrosis factor; tTG, tissue transglutaminase.
Many of the sequential steps in CD pathogenesis are well-elucidated; hence, multiple well-defined targets for research and drug development are available ( Table 1 ). Likewise, therapies focused on the regulation of the immunostimulatory effects have been described for other related pathologies, and due to their efficacy, their indications have been extended to CD.
Summary of strategies for CD grouped according to their goals.
| Strategy | Goal | Therapy | References | |
|---|---|---|---|---|
| Removal of toxic gluten peptides before reaching the intestine | Genetic modification of gluten-containing cereals | Genetically modified wheat flours | [ , , ] | |
| Microbial gluten modification | Pretreatment with probiotic bacteria of the genus (VSL#3) | [ ] | ||
| Pretreatment with microbial transglutaminase (m-TG) and N-methyl-lysine | [ ] | |||
| Masking of antigenic gluten capacity | Polymeric resins HEMA-co-SS | [ , ] | ||
| AGY-010 | [ ] | |||
| Luminal gluten detoxification | Prolyl endopeptidases (PEPs) | (FM-PEP) | [ , ] | |
| (MX-PEP) | [ ] | |||
| (SC-PEP) | [ , ] | |||
| (AN-PEP) | [ ] | |||
| Gluten hydrolytic enzyme cocktail | SC-PEP and EPB-2 (ALV003) | [ ] | ||
| FM-PEP and EPB-2 | [ ] | |||
| Subtilisin derived from (Sub-A) | [ ] | |||
| Cysteine endopeptidase derived from (EP-B2) | [ ] | |||
| Elastase derived from (CEL-3B) | [ ] | |||
| Regulation of the immunostimulatory effects of toxic gluten peptides | Immune response regulation | Inhibition of transglutaminase (ZED 1227) | [ ] | |
| Blocker of HLA DQ binding to T-cells | [ ] | |||
| NK lymphocyte activation blocker: NKG2D receptor antagonists | [ ] | |||
| Lymphocyte recruitment blocker | Anti-α4 integrin (natalizumab) | [ ] | ||
| Anti-integrin α4β7 (vedolizumab) | ||||
| Binding inhibitors CD40-CD40L | ||||
| Binding inhibitors CXCL10- CXCR3 | ||||
| Binding inhibitors CCL25-CCR9 | ||||
| Anti-cytokines | Anti-IL-15, PRV-015, CALY-002 (AMG714) | [ , ] | ||
| Anti-TNF-α (infliximab and adalimumab) | ||||
| Anti-TNF- γ (fontolizumab) | ||||
| Inhibition of the proinflammatory cascade | Anti-inflammatories (generic corticosteroids, budesonide, mesalazine) | [ ] | ||
| Modulation of intestinal permeability | Barrier enhancing therapies | Larazotide acetate (AT-1001 and INN-202) | [ , ] | |
| Immune modulation and induction of tolerance to gluten | Immunomodulation and gluten tolerance | Vaccine Nexvax2 | [ , ] | |
| TAK-101 (CNP-101 and TIMP-GLIA) | [ ] | |||
| KAN-101 | [ ] | |||
| Hookworm infection ( ) | [ ] | |||
| Mucosal tolerance due to genetic modification | [ ] | |||
| Restoration of the imbalance in the gut microbiota | Probiotic supplementation | Microbial therapies | [ , ] | |
TNF, tumor necrosis factor; IgA, immunoglobulin A; Il-15, interleukin 15; NK, natural killer; PEP, prolyl endopeptidase; P-HEMA-co-SS, poly-hydroxyethylmethacrylateco-styrene sulfonate.
3.1. Removal or Reduction of Toxic Gluten Peptides
Therapies aimed at eliminating or reducing gluten peptides can act in food before marketing, during digestion in the human tract, or masking the antigenic capacity before reaching the intestinal mucosa.
3.1.1. Genetic Modification of Gluten-Containing Cereals
The development of cereals with reduced or absent immunogenic gluten proteins is important for the management of CD. The wheat variants currently used have been reported to be more immunogenic than the ancestral or wild variants such as those belonging to the genera Tritordeum or Triticum [ 92 , 93 ]. Genetic advances in plants have successfully allowed the production of wheat lines with very low or completely lacking gluten content through the hybridization of wheat species [ 94 ]. A recent study described the traditional breeding and characterization of a novel ultralow gluten barley variety in which the gluten content was reduced to below 5 ppm by combining three recessive alleles, which act independently to lower the hordein content in the parental varieties [ 59 ].
RNA interference to silence the expression of gluten proteins that contain immunogenic epitopes for CD has been employed as a genetic engineering strategy [ 95 ]. This approach has allowed the development of wheat lines that contain very few immunogenic epitopes of CD, and, therefore, could be consumed by patients with non-celiac gluten sensitivity, since it produces no adverse clinical symptoms [ 96 , 97 ]. Currently, several studies are in progress to understand the effects of these new lines in patients with CD.
The use of CRISPR/Cas9 (Clustered Regulatory Interspaced Palindromic Repeats associated protein 9) technology can precisely and efficiently reduce the amount of α-gliadin in the seed kernel, providing bread and durum lines with reduced immunoreactivity for the celiac community [ 60 , 94 ]. However, it is likely that the deleted gliadin genes need to be replaced by non-immunogenic gliadin variants to obtain adequate elasticity. Additionally, governmental regulations for genetic modification of food products require expensive and time-consuming food safety assessments to be met before product marketing [ 94 ].
3.1.2. Microbial Gluten Modification
The addition of diverse microorganisms in sourdough for fermentation has been studied because it contains proteases capable of hydrolyzing gluten peptides rich in glutamine and proline residues. Diverse studies using species of the genus Lactobacillus have reported that this baking method could obtain safe breads for celiac patients [ 62 , 98 ]. The well-known probiotic preparation VSL#3 comprises eight strains belonging to the genera Bifidobacterium, Lactobacillus, and Streptococcus . This cocktail was assayed during the food processing step and produced tolerable predigested gliadins without α-gliadin peptides p62-75 and 33-mer, but with the palatability of gluten-free products [ 99 ]. This study demonstrated the improvement in the symptoms of adult CD patients with irritable bowel syndrome (IBS) [ 100 ]. Furthermore, the probiotic preparation was capable of stabilizing intraepithelial junctions, promoting the barrier effect that prevents the entry of toxic peptides into the lamina propria [ 91 , 101 ]. However, individual probiotic strains are inadequate to break down gliadin compared to the group efficacy [ 101 , 102 ].
Another investigated approach in the preclinical phase consists of the pretreatment of flours or sourdoughs with microbial transglutaminase (m-TG) and N-methyl-lysine [ 103 , 104 ]. The use of N-methyl lysine and m-TG derived from Streptomyces mobaraensis provoked gluten modification and loss of affinity for the HLA-DQ2 molecule, which leads to less activation of intestinal T lymphocytes [ 105 ]. Although the effect of standard bakery concentrations of microbial transglutaminase (m-TG) in wheat bread preparation on the immunoreactivity of sera of CD patients was investigated, its use in food preparation remains a subject of debate [ 63 ].
3.1.3. Masking of Antigenic Gluten Capacity
The gluten-binding polymer BL-7010 or copolymer poly-hydroxyethylmethacrylate-co-styrene sulfonate (P-HEMA-co-SS) complex is a non-absorbable synthetic origin blocking agent that binds intraluminal gluten [ 64 ]. Therefore, digestive enzymes cannot access the cleavage sites, preventing the degradation of immunogenic peptides that are not absorbed by the intestine and do not induce an immune response. The effect of BL-7010 has been investigated in intestinal biopsy samples from patients with CD [ 64 , 65 , 106 ]. Attenuation of the immune response and the high safety profile in animal models were observed; however, this phase II therapy was discontinued in 2017.
Recent studies have developed neutralizing anti-gliadin antibodies extracted from egg yolk (AGY-010). IgY antibodies have shown effectiveness in neutralizing and absorbing gliadin, as well as resistance to stomach conditions [ 66 ]. This therapy is currently in phase II studies and a study is ongoing to evaluate its efficacy and safety in CD patients [ 107 ]. As the use of egg yolk antibodies might be inefficient for large-scale clinical production, parallel recombinant antibody fragments in single-chain format have been produced for the same purpose [ 108 ].
3.1.4. Luminal Gluten Detoxification
Oral enzyme therapy is focused on the inactivation of gluten peptides in the human gastrointestinal tract before reaching the intestine. Gluten-degrading enzymes seem to hold the most promise as attractive therapies for helping patients with CD to avoid accidental gluten ingestion and to promote better overall health. A prerequisite is that such enzymes should be active under gastro-duodenal conditions, quickly neutralize the T-cell-activating gluten peptides and be safe for human consumption [ 67 , 68 , 70 , 109 ].
Glutenases have been identified in bacteria, fungi, plants, and even insects ( Table 2 ). Although the enzymes studied are endopeptidases, interesting exopeptidases have also been described [ 110 ]. Endopeptidases are further subdivided depending on their catalytic mechanism; among them, prolyl endopeptidases (PEPs) are especially effective in hydrolyzing peptide bonds on the carboxyl side of internal proline residues in gluten-derived oligopeptides [ 69 ]. The potential synergism between gluten-degrading enzymes that differ in their cleavage specificities and optimum pH values raises the possibility of a mixture that would more effectively eliminate the antigenicity of ingested gluten fractions [ 111 ].
Summary of glutenases used in enzyme therapy and classified according to origin of isolation, producer organism, and catalytic mechanism. ND, not determined.
| Source of Enzymes | Peptidase Type | Organism | Isolated Enzyme | References |
|---|---|---|---|---|
| Bacterial peptidases | Prolyl endopeptidase | SC-PEP | [ ] | |
| MX-PEP | [ ] | |||
| FM-PEP | [ ] | |||
| PEP 2RA3 | [ ] | |||
| Subtilisin | ND | [ ] | ||
| Sub-A | [ ] | |||
| ND | [ ] | |||
| Pseudolysin | lasB | [ ] | ||
| Thermolysin | ND | [ ] | ||
| Serine peptidase | ND | [ ] | ||
| ND | GS 188 | ND | [ ] | |
| Serine carboxyl peptidase | A8 | E40 | [ ] | |
| Fungal peptidases | Prolyl endopeptidase | AN-PEP | [ ] | |
| Aspergillopepsin | ASP | [ ] | ||
| Exopeptidase | AO-DPP-IV | [ ] | ||
| Plant peptidases | Cysteine endopeptidase | EP-B2 | [ ] | |
| Caricain | [ ] | |||
| Triticain-α | [ ] | |||
| HvPap-6 CysProt | [ ] | |||
| Insect peptidases | Prolyl peptidase | ND | [ ] | |
| Prolidase | ND | [ ] | ||
| Human peptidases | Elastase | CEL3B | [ ] | |
| CEL2A | [ ] | |||
| Carboxypeptidase | CBPA1 | [ ] |
Among the bacterial enzymes capable of degrading gluten, PEPs are produced by F. meningosepticum [ 68 , 69 ], S. capsulata [ 70 , 71 ] and M. xanthus [ 69 ]. These three enzymes showed high specificity against reference chromogenic substrates and the potential to successfully degrade the immunogenic sequences of gluten. The cysteine endoprotease EP-B2 and PEP from F. meningosepticum complement each other in terms of their gluten hydrolytic properties; however, significant efforts have been made to increase their thermostability to be suitable for industrial applications [ 111 ].
Fungal PEP from A. niger , known as AN-PEP, exhibits post-proline cleavage activity and is highly efficient in degrading gluten [ 72 ]. A clinical study with Tolerase G, an AN-PEP-based supplement, reduced the amount of gluten exposed in the duodenum efficiently, despite not completely degrading the gluten [ 72 ]. The enzyme preparation consisting of AN-PEP from A. niger and DPP-IV from A. oryzae (STAN 1) administered orally in celiac patients appeared to be modest because of the non-specificity of AN-PEP and the very limited proteolytic effect of DPP-IV. Therefore, these studies were stalled in phase II in 2017. In the genus Aspergillus, another enzyme was detected with gluten-degrading activity, termed aspergillopepsin (ASP) from A. niger, although ASP needs to be used as a complementary enzyme because of its incomplete degradation [ 118 ]. In this sense, a dietary supplement has been widely used in the food and feed industry containing ASP from A. niger and DPP-IV from A. oryzae , which successfully degraded small amounts of gluten in vitro [ 119 ].
As previously argued, the combination of enzymes appears to be a future direction in enzyme therapy. The enzymatic cocktail, latiglutenase or IMGX-003 (formerly ALV003), consists of a 1:1 combination of cysteine endoprotease from barley EP-B2 (IMGX-001), and PEP from S. capsulate SC-PEP (IMGX-002). A phase II gluten challenge to investigate its effect on both mucosal and symptomatic protection in CD patients is in progress. Initial findings with latiglutenase have been shown to mitigate gluten-induced intestinal mucosal injury as well as to reduce the severity and frequency of symptoms in patients with CD [ 73 , 125 ]. Evidence of symptom relief was particularly pronounced in patients with positive serology despite following a GFD [ 61 , 126 , 127 ].
An engineered synthetic gluten-degrading enzyme, KumaMax, with technological improvements, is being studied. KumaMax showed similar in vitro results to IMGX-003, although it is still under development [ 128 ]. The gluten-degrading enzyme subtilisin-A (Sub-A) from B. licheniformis was modified by PEGylation and subjected to microencapsulation. The effectiveness was confirmed in vitro and in vivo and showed a significant increase in protection against acid exposure [ 113 ].
Investigating the effect of glutenases on the symptoms and biomarkers in CD patients with randomized, placebo-controlled studies is mandatory; however, this is not as straightforward as it might seem.
3.2. Immune Response Regulation
As inflammatory mediators are common in CD and other gastrointestinal pathologies, certain therapies aimed at avoiding chronic gastrointestinal inflammation could be applied in CD.
tTG plays a critical role in the pathogenesis of CD through the deamidation and transamidation of gluten peptides, which leads to an immune response with inflammation of the intestinal mucosa [ 129 , 130 ]. Hence, the inhibition of tTG results in the abolishment of gluten peptide presentation by HLA-DQ2/DQ8, preventing the immune response. Three varieties of tTG-2 inhibitors have been well described, namely, irreversible inhibitors, reversible inhibitors, and competitive amine inhibitors. ZED-1227 is a highly specific orally active irreversible inhibitor with promising preliminary preclinical results. A phase II clinical study with ZED-1227 is ongoing in EU countries in healthy volunteers [ 76 ]. Nevertheless, tTG plays a critical role in gut wound healing, and its safety and efficacy require further study [ 131 ]. Among competitive inhibitors, cystamine is currently the only competitive commercially available tTG-2 inhibitor despite that it has not been explored for its potential role in CD. Recently, Palansky et al. [ 132 ] discovered that disulfiram, an FDA-approved drug for alcohol abuse, is also a tTG inhibitor. This is the first clinically approved compound to show human tTG inhibitory activity, raising further interesting possibilities for the future in terms of tTG inhibition as a therapeutic strategy in CD [ 133 ].
Another attractive therapeutic target to prevent the activation of the immune response is the HLA-DQ2 blocker. Gluten-like molecules in which proline residues have been replaced by azidoprolines do not elicit an immune response in T-cells isolated from individuals with CD [ 8 ]. Cyclic and dimeric peptides have also been developed that bind DQ2, partially blocking T-cell proliferation and antigen presentation. However, these molecules do not fully block the activation of T-cells; therefore, other nontoxic antagonists with high affinity are currently being studied [ 129 ].
Some studies have highlighted the role of IL-15 and the receptor activator NKG2D and other immune soluble factors as targets of CD treatment. IL-15 plays a critical role in the activation of intraepithelial lymphocytes and participates in both innate and adaptive responses. NKG2D is the receptor of T-cells and natural killer cells [ 134 ]. The first monoclonal antibody (moAb) studied against the IL-15 receptor was Hu-Mik-Beta-1, and positive results were obtained in refractory CD. However, this therapy was stuck in phase I. Second, PRV-015 (also known as AMG 714) is a fully human moAb that has emerged as a leading investigational candidate for nonresponsive CD (NRCD), in which patients maintain disease activity despite an ongoing GFD. Phase II studies have shown a reduction in inflammation and symptoms in a clinical trial with patients with refractory CD type 2 [ 80 ]. Lastly, CALY-002 is a moAb whose safety, tolerability, pharmacokinetics, and pharmacodynamics are being evaluated in phase II studies in both CD and eosinophilic esophagitis [ 135 ].
Tumor necrosis factor (TNF)-γ secreted by T-cells in response to gluten is another therapeutic target under study. Fontolizumab was initially developed for inflammatory bowel disease (IBD) treatment and has been proposed for CD, although clinical trials for this indication have not yet been registered. Infliximab and adalimumab moAbs targeting TNF-α have been used in clinical practice for IBD and could be useful in treating CD [ 76 , 136 ].
Among T-cell-targeted therapies aimed at blocking lymphocyte recruitment, natalizumab is an anti-α4 used in Crohn’s disease and could be useful in CD, although its side effects are very high [ 79 , 137 ]. Vedolizumab is scheduled to start phase II studies that block α4β7 integrin [ 138 ]. In addition, chemokine receptor inhibitors such as CXCR3 and its specific ligands CXCL10 and CXCL11 have also been studied [ 79 ]. These molecules are among the main determinants in the recruitment of immune cells to the intestinal lamina propria and are involved in the uptake of lymphocytes in the presence of gliadin peptides. CCL25 and its receptor CCR9 appear to be a therapeutic alternative in the future, although to date it has only been studied in animal models with Crohn’s disease [ 139 , 140 ].
Anti-inflammatory drugs such as corticosteroids and budesonide are generally used to treat the symptoms of refractory CD. Likewise, mesalazine has been proposed, although it must be remembered that most of these formulations are prepared to be released in the colon and the inflammation in CD affects the small intestine [ 66 ]. Recent studies have shown that mesalazine has a beneficial effect on the molecules and biological mediators of inflammation that occur in the mucosa of celiac patients [ 81 ].
3.3. Barrier Enhancing Therapies
Increased intestinal permeability has been implicated in CD due to both transcellular and paracellular epithelial permeability, with apical junctional protein complexes called tight junctions being key components in the latter process [ 141 ].
Larazotide acetate, formerly known as AT-1001 or INN-202, is a locally acting octapeptide with a sequence analogous to a portion of Vibrio cholerae zonula occludens toxin [ 141 ]. In cultured intestinal epithelial monolayers, larazotide acetate enhanced actin rearrangement and prevented the disassembly of tight junctions [ 142 , 143 ]. In addition, larazotide acetate prevents the passage of gluten peptides to the lamina propria by closing the intercellular junctions of the enterocytes, which could help prevent the development of the immune cascade in celiac patients. Therefore, larazotide acetate is the most advanced experimental drug, showing a reduction in symptoms as well as a reduction in anti-tTG antibody levels. Three phase II studies of larazotide acetate have been completed and published in CD patients undergoing a gluten challenge, but only an excellent safety profile and efficacy with low dose have been reported in patients with NRCD. Therefore, larazotide acetate has moved forward to a phase III registration study for this indication [ 82 , 83 , 144 ].
3.4. Immunomodulation and Gluten Tolerance
Vaccine therapy is the preferred option among alternative treatments to a GFD in patients with CD. It is based on immunization with gluten epitopes, which induces the expansion of regulatory T-cells, restoring oral tolerance to gluten [ 145 ]. The Nexvax2 vaccine (ImmusanT, Cambridge, MA, USA) comprises the use of three gluten epitopes chosen based on a study by Tye-Din et al. [ 145 ]. This study examined epitopes within wheat, barley, and rye with the ability to induce and stimulate T-cells isolated from the serum of patients with CD on a gluten-containing diet. Nexvax2 is one of several CD drugs that has reached phase II clinical trials [ 141 ]. However, although Nexvax2 showed a good safety profile, its efficacy has yet to be demonstrated. Nexvax2 is specific only for individuals with the HLA-DQ2 genotype. Therefore, another vaccine should be investigated in patients with HLA-DQ8 genotyping [ 84 , 85 ].
Biodegradable nanoparticles encapsulated with gliadin proteins TAK-101 (formerly known as CNP-101 and TIMP-GLIA) seem to be a first-in-class agent that induces antigen-specific immune tolerance to CD [ 141 ]. TAK-101 binds inflammatory cells to initiate tolerogenic immune reprogramming. According to the clinicaltrials.gov, the phase II developmental trial of TAK-101 for treating patients with CD was estimated to be completed in July 2019, but it is still in the active phase, not the recruiting phase [ 146 ].
A new therapy in phase I focuses on restoring normal immune tolerance by targeting specific receptors in the liver, named KAN-101 [ 141 ]. The tolerogenic nanoparticles for intravenous injection trigger a cascade of events that drive the re-education of T-cells so that they do not respond to gluten antigens [ 87 ].
The administration of N. americanus infective larvae in patients with CD interferes with the host immune response due to its survival in the intestine. Studies of duodenal biopsies from CD individuals infected with N. americanus and exposed to gluten have shown a reduction in the production of IL-2, IFN-γ, and IL-17. In addition, the absence of histological lesions and even a decrease in anti-tTG antibody levels have been demonstrated [ 88 ]. N. americanus is currently in phase II clinical trials, although problems with CD patient acceptance for routine clinical use are anticipated [ 66 , 147 ].
Finally, other studies based on the tolerance of the mucosa to genetic modification are in the initial phase of investigations. These studies specifically focused on organoids derived from the human intestine, providing a model to study the response to gluten and the effects of molecules derived from the microbiota in patients with CD [ 89 ].
3.5. Restoration of the Imbalance in the Gut Microbiota
The gut microbiota is involved in the initiation and perpetuation of intestinal inflammation in several chronic diseases. Indeed, several studies have identified certain microorganisms in CD patients and healthy subjects. Therefore, alteration of the microbiota could play a significant role in the pathogenesis of CD. Recent studies have focused on the role of the gut microbiota in CD and the complex relationship between its composition, genetic background, GFD, and persistence of clinical symptoms [ 90 , 148 ]. The specific mechanisms by which microorganisms can participate in the development of responses to gluten are broad and include the metabolism of trigger antigen responses, enhancement of the intestinal barrier, and modulation of adaptive and innate immune responses [ 149 ].
Recent data have shown that genetics (HLA-DQ-2 or DQ-8) may predispose individuals with CD to dysbiosis [ 90 , 148 ]. Palma et al. [ 150 ] studied the effects of following a GFD on the composition of gut microbiota in healthy subjects. A significant decrease of Bifidobacterium , Clostridium lituseburense , and Faecalibacterium prausnitzii and an increase in Enterobacteriaceae and Escherichia coli counts were found. Therefore, the supplementation with a probiotic to restore the imbalance in the gut microbiota might be a reasonable therapeutic option by downregulating the proinflammatory immune response in CD patients [ 90 ]. The design of specific probiotics comprises advanced genomic and metabolomics techniques using the interactions between the human body-microbiota and intra-microbiota, eventually leading to tailored specific probiotic therapies for microbiome regulation and health sustainability.
Probiotics play an important role in preventing the overgrowth of potentially pathogenic bacteria and maintaining the integrity of the gut mucosal barrier. The beneficial effects of probiotics have been previously studied in adult patients with IBS. Oral administration of a probiotic mixture of Lactobacillus plantarum 14D-CECT 4528, Lactobacillus casei, Bifidobacterium breve Bbr8 LMG P-17501, B. breve Bl10 LMG P-17500, and Bifidobacterium animalis under randomized, double-blind, and placebo-controlled conditions showed the improvement in symptoms of adult CD patients with IBS [ 100 ]. In the future, microorganisms or even genetically engineered microorganisms could be used to act as living enzyme machinery as well as vectors for the delivery of endopeptidases capable of digesting gluten in the stomach, thereby allowing celiac patients to have a controlled dietary gluten intake [ 91 , 151 ].
In conclusion, probiotics are not expected to provide a rapid cure for complex diseases such as CD, but rather to alleviate the severity of symptoms [ 99 ]. More studies are needed to address how the gut microbiome can modulate or alter the course of the disease. To date, there are no guidelines available that recommend probiotic use in patients with CD. However, the data suggest a strong adjunctive role in the management of symptoms and bacterial overgrowth.
4. Clinical Trials
Clinical endpoints are variables to quantify the potential effect of the treatment or intervention under study and reflect or characterize how a subject “feels, functions, or survives” [ 152 ]. Many major disease areas have established clinical trial endpoints because a fair number of registration trials have already been conducted and drugs approved for marketing. As in CD, there are no approved products and little experience, and agreed endpoints are lacking. Certain treatments in CD could control symptoms and prevent worsening of damage, while others are, at least initially, focused primarily on healing and maintenance of healing, with little effect on symptoms. Therefore, different endpoints or endpoint instruments are needed [ 153 ]. To date, only larazotide acetate is currently in phase III studies; most of them at phase II and a few phase I trials have explored its efficacy. Some therapies are being evaluated in preclinical trials and are postulated to be promising treatments for CD pathogenesis ( Figure 3 ). We are facing many promising and emerging options for the treatment of CD.

Clinical and preclinical development pipeline for CD. CD, celiac disease; PEP, prolyl endopeptidases; TNF, tumor necrosis factor; tTG, tissue transglutaminase.
5. Conclusions
Although a GFD has been shown to be safe and effective in most celiac patients, the limitations caused by dietary gluten restriction and high gluten exposure rates raise the need to develop new therapies for CD. Different non-dietary therapeutic strategies are currently in the development phase and in clinical research, which could be a useful option in the medium- or long-term in patients with CD. To date, larazotide acetate is the most advanced experimental drug that has shown a reduction in symptoms as well as anti-tTG antibody titers. Promising PRV-015 immunotherapy requires more assays to establish rational targets for disease prevention. The use of glutenases as food preprocessors has proven to be very effective; however, the use of oral glutenases is perhaps the most accepted strategy for patients with CD and one of the most numerous options in terms of ongoing studies. All efforts are now being made to assess the effectiveness of these enzymes as a supplement to a GFD, highlighting the phase II results of IMGX-003 being very promising. Vaccine therapy has limitations, such as that it can engage only known or previously investigated immunogenic epitopes and effectiveness with the specific HLA-DQ2 genotype. However, if successful, it has the potential to have prolonged benefits on patients.
In addition, other many interesting drugs are in early research stages, such as tTG inhibitors, HLA blockers, and probiotics, although probiotics will probably need to be combined with long-term dietary changes. While several trials are ongoing or underway for CD, there is no consensus on outcome measures in CD patient trials.
Preventing the onset of CD entirely would be the most beneficial and desirable approach; however, recent approaches argue whether ingesting certain amounts of gluten plays a complementary or “adjuvant” role to a GFD and not as a substitute to a GFD in patients with CD. Nevertheless, some of these therapies could also be effective in other gluten-related pathologies in which a minimal amount of gluten is tolerable.
Great efforts are ongoing to determine the effectiveness and the dose limit of gluten ingested in these therapies. It is also obvious that the possibility of using synergistic strategies could increase the maximum safe doses allowed for CD; therefore, this issue will be the next challenge.
Author Contributions
Conceptualization, V.S. and M.d.L.M.; data curation, V.S. and M.d.L.M.; formal analysis, V.S. and M.d.L.M.; investigation, V.S., Á.R.-C., C.S. and M.d.L.M.; methodology, V.S. and M.d.L.M.; resources, V.S. and M.d.L.M.; writing—original draft, V.S., Á.R.-C., C.S. and M.d.L.M.; writing—review and editing, V.S., Á.R.-C., C.S. and M.d.L.M. All authors have read and agreed to the published version of the manuscript.
This work was supported by grant from Federación de Asociaciones de Celíacos de España (FACE) (SUBN/2019/005).
Conflicts of Interest
The authors have no potential conflict of interest to declare. None of the authors have any conflicts of interest that could affect the performance of the work or the interpretation of the data.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
New treatment may reverse celiac disease
- Feinberg School of Medicine
Results of a new phase 2 clinical trial using technology developed at Northwestern Medicine show it is possible to induce immune tolerance to gluten in individuals with celiac disease. The findings may pave the way for treated celiac patients to eventually tolerate gluten in their diet.
After treatment with the technology, the patients were able to eat gluten with a substantial reduction in inflammation. The results also show a trend toward protecting patients’ small intestine from gluten exposure.
The findings were presented as a late-breaking presentation Oct. 22 at the European Gastroenterology Week conference in Barcelona, Spain.
The technology is a biodegradable nanoparticle containing gluten that teaches the immune system the antigen (allergen) is safe. The nanoparticle acts like a Trojan horse, hiding the allergen in a friendly shell, to convince the immune system not to attack it.
Beyond celiac disease, the finding sets the stage for the technology — a nanoparticle containing the antigen triggering the allergy or autoimmune disease — to treat a host of other diseases and allergies including multiple sclerosis, type 1 diabetes, peanut allergy, asthma and more.
The technology was developed in the lab of Stephen Miller, professor of microbiology and immunology at Northwestern University Feinberg School of Medicine, who has spent decades refining the technology.
“This is the first demonstration the technology works in patients,” said Miller, the J udy Gugenheim Research Professor of Microbiology and Immunology. “We have also shown that we can encapsulate myelin into the nanoparticle to induce tolerance to that substance in multiple sclerosis models, or put a protein from pancreatic beta cells to induce tolerance to insulin in type 1 diabetes models.”
When the allergen-loaded nanoparticle is injected into the bloodstream, the immune system isn’t concerned with it, because it sees the particle as innocuous debris. Then the nanoparticle and its hidden cargo are consumed by a macrophage, essentially a vacuum-cleaner cell that clears cellular debris and pathogens from the body.
“The vacuum-cleaner cell presents the allergen or antigen to the immune system in a way that says, ‘No worries, this belongs here,’” Miller said. “The immune system then shuts down its attack on the allergen, and the immune system is reset to normal.”
In the celiac disease trial, the nanoparticle was loaded with gliadin, the major component of dietary gluten, found in cereal grains such as wheat. A week after treatment, the patients were fed gluten for 14 days. Without treatment, celiac patients eating gluten developed marked immune responses to gliadin and damage in their small intestine. Celiac patients treated with the COUR nanoparticle, CNP-101, showed 90% less immune inflammation response than untreated patients. By stopping the inflammatory response, CNP-101 showed the capacity to protect the intestines from gluten related injury.
There currently is no treatment for celiac disease.
“Doctors can only prescribe gluten avoidance, which is not always effective and carries a heavy social and economic toll for celiac patients,” Miller said.
About 1% of the population has celiac disease, a serious autoimmune disease in which the ingestion of gluten leads to damage in the small intestine. When people with celiac disease eat gluten (a protein found in wheat), their body mounts an immune response that attacks the small intestine.
Autoimmune diseases generally can only be treated with immune suppressants that provide some relief, but undermine the immune system and lead to toxic side-effects. CNP-101 does not suppress the immune system but reverses the course of disease.
“Celiac disease is unlike many other autoimmune disorders because the offending antigen (environmental trigger) is well known -- gluten in the diet,” said Dr. Ciaran Kelly, professor of medicine at Harvard Medical School and director of the Celiac Center at Beth Israel Deaconess Medical Center. “This makes celiac disease a perfect condition to address using this exciting nanoparticle induced immune tolerance approach.”
Kelly, who will be presenting the research abstract in Barcelona, has been working with Miller to apply the technology and define the therapeutic approach to treating celiac disease.
The nanotechnology was licensed to COUR Pharmaceuticals Co., a biotech based in Northbrook and co-founded by Miller. COUR developed CNP-101, which was granted Fast Track status from the U.S. Food and Drug Administration, and brought the therapy to patients in collaboration with Takeda Pharmaceuticals. Takeda will announce Tuesday they have acquired an exclusive global license to develop and commercialize this investigational medicine for celiac disease.
“Given the license by Takeda, COUR will focus on clinical programs in peanut allergy and multiple sclerosis in the near term and broaden even further over time,” said John J. Puisis, president and chief executive officer of COUR.
Miller, who is on the COUR scientific advisory board, is a stock grantee and a paid consultant for the company. Northwestern University has a financial interest in COUR.
Editor’s Picks

Deering Library undergoing major renovations
Northwestern celebrates the groundbreaking of new ryan field, forget imitation crab — researchers test snackable snails, related stories.
New biomaterial regrows damaged cartilage in joints
Older adults in illinois at increased risk for suicide, ‘dancing molecules’ heal cartilage damage.
- Open access
- Published: 23 July 2019
Celiac disease: a comprehensive current review
- Giacomo Caio ORCID: orcid.org/0000-0002-4244-4529 1 , 2 na1 ,
- Umberto Volta 3 na1 ,
- Anna Sapone 2 , 4 ,
- Daniel A. Leffler 4 , 5 ,
- Roberto De Giorgio 1 ,
- Carlo Catassi 2 , 6 na2 &
- Alessio Fasano 2 na2
BMC Medicine volume 17 , Article number: 142 ( 2019 ) Cite this article
134k Accesses
540 Citations
159 Altmetric
Metrics details
Celiac disease remains a challenging condition because of a steady increase in knowledge tackling its pathophysiology, diagnosis, management, and possible therapeutic options.
A major milestone in the history of celiac disease was the identification of tissue transglutaminase as the autoantigen, thereby confirming the autoimmune nature of this disorder. A genetic background ( HLA-DQ2/DQ8 positivity and non-HLA genes) is a mandatory determinant of the development of the disease, which occurs with the contribution of environmental factors (e.g., viral infections and dysbiosis of gut microbiota). Its prevalence in the general population is of approximately 1%, with female predominance. The disease can occur at any age, with a variety of symptoms/manifestations. This multifaceted clinical presentation leads to several phenotypes, i.e., gastrointestinal, extraintestinal, subclinical, potential, seronegative, non-responsive, and refractory. Although small intestinal biopsy remains the diagnostic ‘gold standard’, highly sensitive and specific serological tests, such as tissue transglutaminase, endomysial and deamidated gliadin peptide antibodies, have become gradually more important in the diagnostic work-up of celiac disease. Currently, the only treatment for celiac disease is a life-long, strict gluten-free diet leading to improvement in quality of life, ameliorating symptoms, and preventing the occurrence of refractory celiac disease, ulcerative jejunoileitis, and small intestinal adenocarcinoma and lymphoma.
Conclusions
The present review is timely and provides a thorough appraisal of various aspects characterizing celiac disease. Remaining challenges include obtaining a better understanding of still-unclear phenotypes such as slow-responsive, potential (minimal lesions) and seronegative celiac disease. The identification of alternative or complementary treatments to the gluten-free diet brings hope for patients unavoidably burdened by diet restrictions.
Peer Review reports
Introduction
Celiac disease (CD) is an autoimmune condition characterized by a specific serological and histological profile triggered by gluten ingestion in genetically predisposed individuals [ 1 ]. Gluten is the general term for alcohol-soluble proteins present in various cereals, including wheat, rye, barley, spelt, and kamut [ 1 ]. In recent years, there have been significant changes in the diagnosis, pathogenesis, and natural history of this condition [ 2 ], with CD undergoing a true ‘metamorphosis’ due to the steady increase in the number of diagnoses identified, even in geriatric patients [ 2 ]. This has been mainly attributed to the greater availability of sensitive and specific screening tests, which allow identification of the risk groups for CD and led to a significant raise in diagnoses worldwide [ 2 , 3 , 4 , 5 ]. Several theories have suggested that the globalization and ubiquitous spread of ‘false’ or ‘extreme’ versions of the Mediterranean diet including the consumption of very high quantities of gluten (up to 20 g/day), has led to an increased prevalence and incidence of CD [ 3 , 4 ]. In addition, the quality of gluten itself might also play a contributory role. Indeed, the production of new grain variants due to technological rather than nutritional reasons may have influenced the observed increase in the number of CD diagnoses in recent years [ 4 , 5 ]. However, these hypotheses have not been confirmed and the real cause of the risk in CD diagnoses remains unknown. Furthermore, the epidemiological observation that similar ‘epidemics’ are reported for other autoimmune diseases in the Western hemisphere [ 6 ] suggests that environmental factors other than gluten can be at play.
In this article, we aimed to provide a thorough review on the multifaceted features of CD spanning from its epidemiological, pathogenetic, clinical, and diagnostic aspects to therapeutic strategies using a practical approach in order to help general practitioners, internal medicine physicians, and gastroenterologists in their clinical practice.
- Epidemiology
CD is one of the most common autoimmune disorders, with a reported prevalence of 0.5–1% of the general population (Table 1 ), with the exception of areas showing low frequency of CD-predisposing genes and low gluten consumption (e.g., sub-Saharan Africa and Japan) [ 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. Studies have shown that most CD cases remain undetected in the absence of serological screening due to heterogeneous symptoms and/or poor disease awareness. CD prevalence is increasing in Western countries. Between the years 1975 and 2000, CD prevalence increased 5-fold in the US, for reasons that are currently unknown [ 14 ]. The prevalence of CD is higher in first-degree CD relatives (10–15%) and in other at-risk groups, particularly patients with Down syndrome, type 1 diabetes, or IgA deficiency [ 1 ].
Pathophysiology
CD is a unique autoimmune disease in that its key genetic elements (human leukocyte antigen (HLA)-DQ2 and HLA-DQ8), the auto-antigen involved (tissue transglutaminase (tTG)), and the environmental trigger (gluten) are all well defined. A major drawback in CD research has been the lack of a reliable and reproducible animal model, with the possible exception of the Irish setter dog, which may develop a gluten-related disease [ 15 ]. Nevertheless, new technologies pertinent to human gut biology and immunology are opening unprecedented opportunities for major research breakthroughs.
As with many other autoimmune diseases, we have witnessed an epidemic of CD, questioning the previous paradigm that gluten is the only key element dictating the onset of the disease in genetically at-risk subjects. Improved hygiene and lack of exposure to various microorganisms also have been linked with a steep increase in autoimmune disorders in industrialized countries during the past 40 years [ 1 , 16 ]. The hygiene hypothesis argues that the rising incidence of many autoimmune diseases may partially be the result of lifestyle and environmental changes that have reduced our exposure to pathogens. With breakthroughs in the role of the gut microbiological ecosystem [ 17 ] in dictating the balance between tolerance and immune response leading to autoimmunity, this hypothesis is under scrutiny. Regardless of whether autoimmune diseases are due to too much or too little exposure to microorganisms, it is generally accepted that adaptive immunity and imbalance between T helper 1 and 2 cell responses are key elements of the pathogenesis of the autoimmune process. Besides genetic predisposition and exposure to gluten, loss of intestinal barrier function, a pro-inflammatory innate immune response triggered by gluten, inappropriate adaptive immune response, and an imbalanced gut microbiome all seem to be key ‘ingredients’ of the CD autoimmunity recipe.
As with any other autoimmune disease, CD has a strong hereditary component as testified by its high familial recurrence (~ 10–15%) and the high concordance of the disease among monozygotic twins (75–80%) [ 18 ]. Also common to other autoimmune diseases is the relevant role of HLA class II heterodimers, specifically DQ2 and DQ8, in the heritability of CD. HLA-DQ2 homozygosis confers a much higher risk (25–30%) of developing early-onset CD in infants with a first-degree family member affected by the disease [ 19 , 20 , 21 ]. Since HLA-DQ2/HLA-DQ8 is frequent among the general population (25–35%), and only 3% of these HLA-compatible individuals will go on to develop CD [ 22 ], it is not surprising that genome-wide association studies have identified more than 100 non-HLA-related genes associated with CD [ 18 , 23 ]. The relevance of these additional genes in conferring genetic risk for CD is rather limited, but they may lead to the discovery of key pathways potentially involved in disease pathogenesis.

Gluten as an environmental trigger of CD
Introduced 10,000 years ago during the transition from a nomadic lifestyle to agricultural settlements, gluten-containing grains are a recent addition to the human diet. Moreover, gluten is one of the few digestion-resistant proteins consumed chronically in significant quantities and is constituted by several non-digestible immunogenic peptides. These two characteristics could help in breaking the tolerance to this food antigen, when the immune system is activated, as can happen during an enteric infection. Gliadins, key components of gluten, are complex proteins unusually rich in prolines and glutamines and are not completely digestible by intestinal enzymes [ 24 ]. The final product of this partial digestion is a mix of peptides that can trigger host responses (increased gut permeability and innate and adaptive immune response) that closely resemble those instigated by the exposure to potentially harmful microorganisms [ 25 , 26 , 27 , 28 ].
Gluten trafficking from lumen to lamina propria (paracellular and transcellular)
Studies from our group and others have shown that gliadin can cause an immediate and transient increase in intercellular tight junction permeability of intestinal epithelial cells [ 23 , 24 ] (Fig. 1 ). This effect has been linked to the release of zonulin, a family of molecules that increases paracellular permeability by causing tight junction disassembly [ 29 , 30 , 31 ]. Gliadin enhances zonulin-dependent increased gut paracellular permeability irrespective of disease status [ 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ]. Similarly, when tested in C57BL/6 mice duodenal tissues, gliadin caused a myeloid differentiation primary response 88-dependent increase in gut mucosa permeability [ 40 ]. We have also identified two alpha-gliadin motifs that can modulate the intestinal barrier function by binding to chemokine receptor 3, with subsequent zonulin release that causes disassembly of the interepithelial tight junction complex [ 41 ]. The involvement of the paracellular pathway for gluten trafficking in the lamina propria has also been corroborated by genetic studies identifying an association of some tight junction genes with CD [ 42 , 43 , 44 ]. There is solid evidence that gluten can also cross the intestinal barrier through the transcellular pathway once tolerance to gluten has been broken [ 45 , 46 ]. The transferrin receptor CD71, normally expressed on the basolateral side of enterocytes, is overexpressed on the luminal side of the intestinal epithelium in CD patients during the acute phase of the disease, leading to an apical-to-basal retrotranscytosis of gliadin peptides complexed with secretory IgA [ 47 ]. This retrotranscytosis of secretory IgA–gliadin complexes protects gliadin fragments from lysosomal degradation and promotes the entry of harmful gliadin peptides into the intestinal lamina propria [ 47 ], thereby perpetuating intestinal inflammation initiated by the paracellular passage of these peptides (Fig. 1 ). Because of their resistance, the gluten immunogenic peptides (GIP) can cross the defective epithelial lining, reach the blood stream (thus extending the inflammatory process), and finally be excreted with the urine [ 48 ].
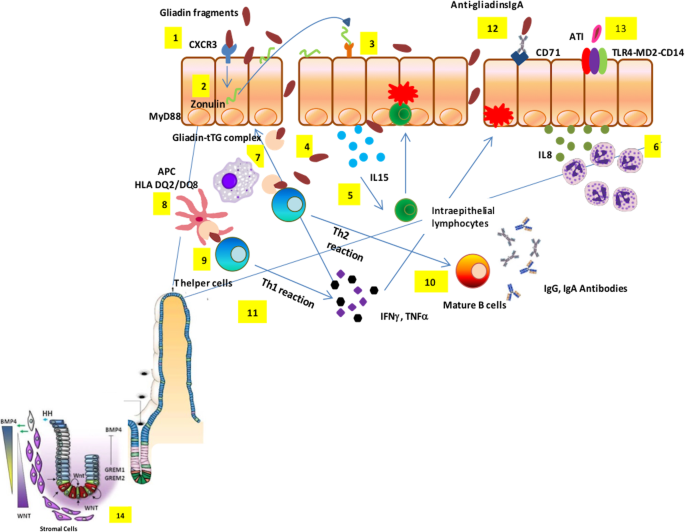
Celiac disease pathogenesis. Partially digested gliadin fragments interact with chemokine receptor 3 on the apical side of epithelium (1) inducing a myeloid differentiation primary response 88-dependent release of zonulin (2). Zonulin interacts with the intestinal epithelium and triggers increased intestinal permeability (3). Functional loss of the gut barrier facilitates gliadin peptide translocation from lumen to the lamina propria (4). Gliadin peptides trigger release of IL-15, keratinocyte growth factor, and IL-8 (5), with consequent recruitment of neutrophils in the lamina propria (6). Simultaneously, alpha-amylase/trypsin inhibitors engage the Toll like receptor 4–MD2–CD14 complex with subsequent up-regulation of maturation markers and release of proinflammatory cytokines (7). Following innate immune-mediated apoptosis of intestinal cells with subsequent release of intracellular tissue transglutaminase, gliadin peptides are partially deamidated (8). Deamidated gliadin is recognized by DQ2/8 + antigen presenting cells (9) and then presented to T helper cells (10). T helper cells trigger activation and maturation of B cells, producing IgM, IgG, and IgA antibodies against tissue transglutaminase (11). T helper cells also produce pro-inflammatory cytokines (interferon γ and tumor necrosis factor α) (12), which in turn further increase gut permeability and, together with T killer cells, initiate the enteropathy. Damaged enterocytes express CD71 transporter also on their apical side, resulting in retrotranscytosis of secretory IgA-gliadin complexes (13), thus potentiating gluten trafficking from gut lumen to lamina propria. Ultimately, the interaction between CD4 + T cells in the lamina propria with gliadin induces their activation and proliferation, with production of proinflammatory cytokines, metalloproteases, and keratinocyte growth factor by stromal cells, which induces crypt hyperplasia and villous blunting secondary to intestinal epithelial cell death induced by intraepithelial lymphocytes. The hyperplastic crypts (14) are characterized by an expansion of the immature progenitor cells compartment (WNT) and downregulation of the Hedgehog signaling cascade. An increased number of stromal cells known to be part of the intestinal stem cell niche and increased levels of bone morphogenetic protein antagonists, like Gremlin-1 and Gremlin-2, may further contribute to the crypt hyperplasia present in celiac disease
The innate immune response
Innate immunity plays a critical role in initiating CD, and cytokines such as interleukin (IL)-15 and interferon α can prime the innate immune response by polarizing dendritic cells and intraepithelial lymphocyte function [ 49 ]. Recent results suggest that specific gliadin peptides may induce epithelial growth factor and an IL-15-dependent proliferation of enterocytes, structural modifications, vesicular trafficking alterations, signaling and proliferation, and stress/innate immunity activation [ 50 ]. Alpha-amylase/trypsin inhibitors – molecules conferring pest resistance in wheat – also seem to play a key role in CD innate immune response by engaging the Toll-like receptor 4–MD2–CD14 complex with subsequent up-regulation of maturation markers and release of proinflammatory cytokines in cells from CD patients [ 51 ]. These mucosal events, along with the functional breach of epithelial barrier function secondary to the gliadin-mediated zonulin release [ 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ], the subsequent access of toxic peptides in the lamina propria, and gliadin-induced production of high levels of the neutrophil-activating and chemoattractant chemokine IL-8 [ 26 , 52 ], cause the ‘perfect storm’ to initiate CD enteropathy (Fig. 1 ). More recently, our group showed that gliadin exerts a direct neutrophil chemoattractant effect by interacting with fMet-Leu-Phe receptor 1 [ 53 , 54 ].
The adaptive immune response
The erroneous adaptive immune response consequence of a highly specific interplay between selected gluten peptides and major histocompatibility complex class II HLA-DQ2/8-antigen restricted T cells plays a paramount role in CD pathogenesis [ 55 ]. Dependent on the post-translational deamidation of gluten peptides by transglutaminase 2 (TG2), this interplay is influenced by the initial imprinting of the innate immune system through IL-15 upregulation that promotes the CD4 + T cell adaptive immune response [ 56 , 57 ]. Presentation of gluten to CD4 + T cells carried out by dendritic cells as well as macrophages, B cells, and even enterocytes expressing HLA class II, can cause their recirculation in the lamina propria [ 58 ]. The contact of CD4 + T cells in the lamina propria with gluten induces their activation and proliferation, with production of proinflammatory cytokines, metalloproteases, and keratinocyte growth factor by stromal cells, which induces cryptal hyperplasia and villous blunting secondary to intestinal epithelial cell death induced by intraepithelial lymphocytes (IELs) [ 58 ]. Additionally, there is an overexpression of membrane-bound IL-15 on enterocytes in active CD causing over-expression of the natural killer (NK) receptors CD94 and NKG2D by CD3 + IELs [ 59 ]. CD crypt hyperplasia has been hypothesized to be the consequence of an imbalance between continuous tissue damage due to the mucosal autoimmune insult described above and inability of the stem cells to compensate. We have recently provided a more mechanistic, evidence-based explanation for hyperplastic crypts in active CD by showing that the celiac hyperplastic crypt is characterized by an expansion of the immature progenitor cell compartment and downregulation of the Hedgehog signaling cascade [ 60 ]. These data shed light on the molecular mechanisms underlying CD histopathology and illuminate the reason for the lack of enteropathy in the mouse models for CD. Indeed, lack of consistent CD-like enteropathy in humanized mice [ 61 ] supports the concept that the accelerated disruption of enterocytes secondary to the adaptive CD4 + T cell insult cannot fully explain CD pathogenesis, supporting the notion that an intrinsic defect of the stem cell compartment in subjects at risk of CD is a key element of CD enteropathy [ 60 , 62 ].
The role of the gut microbiome in the pathogenesis of CD
In Western countries, a rise in the overall prevalence of CD has been well documented, but the reasons for this ‘epidemic’ remain elusive. The combination of epidemiological, clinical, and animal studies suggests that broad exposure to a wealth of commensal, non-pathogenic microorganisms early in life are associated with protection against CD and that pre-, peri-, and post-natal environmental factors may strongly influence the gut ecosystem [ 17 ]. Therefore, the hygiene hypothesis concept can be misleading, while an ‘environment-dependent dysbiosis hypothesis’ would more closely reflect the interplay between host and environmental pressure dictating the balance between health and disease. Several studies have shown an association between CD and a change in the microbiome composition [ 63 , 64 ]. However, these associative studies do not necessarily imply causation between microbiota composition and CD pathogenesis. Many environmental factors known to influence the composition of the intestinal microbiota are also thought to play a role in the development of CD [ 19 , 21 ].
It has been reported that, compared to control infants, neonates at family risk of CD had a decreased representation of Bacteriodetes and a higher abundance of Firmicutes [ 65 ]. This study also showed that infants who developed autoimmunity had decreased lactate signals in their stools coincident with a diminished representation in Lactobacillus species in their microbiome, which preceded the first detection of positive antibodies [ 65 ]. Early microbiota alterations in infants were also suggested in a recent study comparing microbial communities between DQ2 + and DQ2 − infants [ 66 ]. However, to move from association to causation, large-scale, longitudinal studies are necessary to define if and how gut microbiota composition and metabolomic profiles may influence the loss of gluten tolerance and subsequent onset of CD in genetically susceptible subjects.
Clinical presentation
CD is diagnosed more frequently in women with a female-to-male ratio ranging from 2:1 to 3:1 [ 1 , 2 ]. However, based on serological screening, the actual female-to-male ratio is 1.5:1 [ 67 ]. The disease can occur at any age from early childhood to the elderly, with two peaks of onset – one shortly after weaning with gluten in the first 2 years of life, and the other in the second or third decades of life. The diagnosis of CD can be challenging since symptoms can vary significantly from patient to patient [ 68 ].
In 2011, the Oslo classification of CD identified the following clinical presentations: classic, non-classic, subclinical, potential and refractory [ 69 ]. Instead of the ‘classic/non-classic’ categorization, which does not fully reflect current clinical presentations, in this review, we will use a more practical terminology, i.e., intestinal/extraintestinal. These two terms better represent the main clinical phenotypes of CD, which may occur individually (i.e., intestinal vs. extraintestinal) or in combination [ 70 ].
The intestinal form of CD is more commonly detected in the pediatric population and children younger than 3 years and is characterized by diarrhea, loss of appetite, abdominal distention, and failure to thrive [ 71 ]. Older children and adults may complain of diarrhea, bloating, constipation, abdominal pain, or weight loss [ 72 ]. Nonetheless, in adults, the malabsorption syndrome with chronic diarrhea, weight loss and significant asthenia is quite rare. Despite its uncommon detection, this phenotype can cause hospitalization due to cachexia, sarcopenia, significant hypoalbuminemia, and electrolyte abnormalities. Conversely, an irritable bowel syndrome (IBS)-like presentation with constipation or alternating bowel and/or dyspepsia-like symptoms, such as nausea and sometimes vomiting, is more frequent [ 2 ].
Extraintestinal symptoms are common in both children and adults [ 2 , 72 ]. They include iron deficiency microcytic anemia, detectable in up to 40% of cases (by cause of iron malabsorption or chronic inflammation) [ 73 ] or, more rarely, macrocytic anemia due to folic acid and/or vitamin B12 deficiency (more frequent in Europe than in the US). Changes in bone mineral density, including osteopenia or osteoporosis (affecting about 70% of patients at diagnosis), are related to altered absorption of calcium and vitamin D3 [ 74 ]. In children, growth retardation and short stature can raise the suspect of an underlying CD. Other signs include tooth enamel defects, aphthous stomatitis (identified in about 20% of undiagnosed CD patients) [ 75 ], and hypertransaminasemia (40–50% of untreated patients), which can be ascribed to food and bacterial antigen translocation reaching the liver due to increased intestinal permeability [ 76 ]. A wide array of neurological symptoms, such as headache, paresthesia, neuroinflammation, anxiety and depression, can be detectable in CD patients. The clinical presentation may also include changes in reproductive function characterized by late menarche, amenorrhea, recurrent miscarriages, premature birth, early menopause, and changes in the number and mobility of spermatozoa. Notably, these manifestations can be reversed when patients start a strict gluten-free diet (GFD), although fatigue and some neurological manifestation as well as functional gastrointestinal (GI) symptoms can persist for a long period in a subgroup of CD patients [ 2 , 77 , 78 , 79 , 80 , 81 ].
The subclinical form includes patients with symptoms/signs below the clinical identification threshold and are often recognizable only after the appreciation of the beneficial effects induced by the GFD. A typical example of subclinical cases are those patients undergoing antibody screening due to being relatives of CD patients or cases identified as a result of a screening strategy in the general population [ 2 , 69 ]. The prevalence of various CD clinical phenotypes observed in our experience is reported in Fig. 2 .
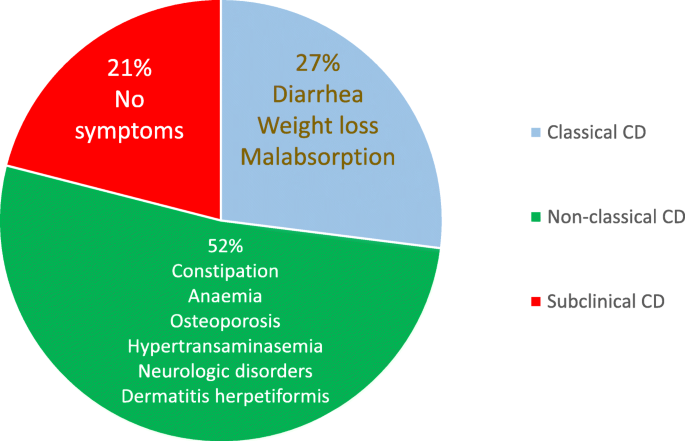
Prevalence of clinical phenotypes of adult celiac disease in our experience
CD can be associated with different autoimmune and idiopathic diseases, including dermatitis herpetiformis (which, as a single manifestation, should prompt testing for CD), type 1 diabetes mellitus, Hashimoto’s thyroiditis, selective IgA deficiency, alopecia areata, Addison’s disease, connective tissue diseases (mainly Sjogren’s syndrome), chromosomal diseases (Down, Turner, and William’s syndromes), neurological diseases (cerebellar ataxia, peripheral neuropathy, epilepsy with and without occipital calcifications), hepatic autoimmune diseases (primary biliary cholangitis, autoimmune hepatitis, primary sclerosing cholangitis), and idiopathic dilated cardiomyopathy (Table 2 ) [ 2 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 ]. The importance of diagnosing CD associated with these concomitant diseases is twofold since a GFD is able to resolve symptoms, prevent complications, and improve some of the CD associated diseases [ 2 ].
The potential form of CD is characterized by positive serological and genetic markers with a normal intestinal mucosa and minimal signs of inflammation such an increase in IELs [ 69 ]. Patients with the potential form can manifest with classic and non-classic symptoms or be entirely asymptomatic. The scientific community has not universally agreed on whether or not a GFD should be prescribed for patients with potential CD.
Finally, refractory CD (RCD) is characterized by persistent symptoms and atrophy of the intestinal villi after at least 12 months of a strict GFD. RCD can lead to complications such as ulcerative jejunoileitis, collagenous sprue, and intestinal lymphoma [ 69 ].
In recent years, other forms of CD (not included in the Oslo Classification [ 69 ]), i.e., seronegative and GFD non-responsive CD, have been identified in the clinical practice. The seronegative form is characterized by the lack of demonstrable serological markers along with clinical signs of severe malabsorption and atrophy of the intestinal mucosa [ 94 ]. This form should be included in the differential diagnosis with other diseases that cause atrophy of the intestinal villi. The term non-responsive CD indicates GI symptoms that persist despite a GFD of more than 12 months [ 95 ]; however, it does not differentiate between active CD and associated conditions, which can be responsible for symptom persistence (Fig. 3 ) and alternative terminology is discussed below.
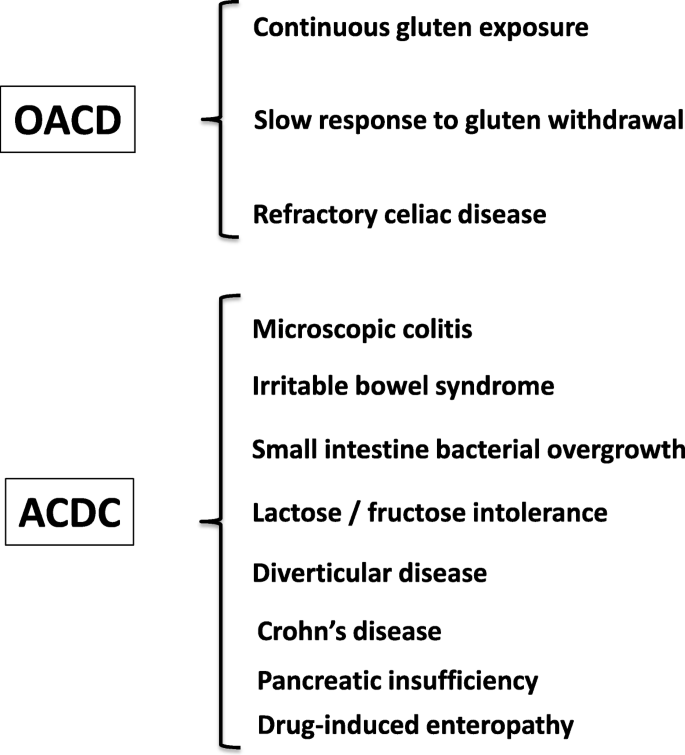
Causes of ongoing signs and/or symptoms of celiac disease (CD) despite a gluten-free diet (formerly referred to as ‘non-responsive’ CD). In this review, two clinical phenotypes have been proposed – ongoing active celiac disease (OACD), related to three main causes, and associated celiac disease conditions (ACDC), encompassing a wide array of diseases
The gold standard for CD diagnosis is represented by the combination of mucosal changes detected by duodenal biopsy and by positivity of serological tests (anti-tTG antibodies, anti-endomysium antibodies (EmA), and deamidated gliadin peptide (DGP) antibodies). Despite the progress made in serology, no antibody test currently available provides a sensitivity and specificity of 100% (Table 3 ) [ 96 , 97 ], thus requiring intestinal biopsy as a key adjunct for establishing a correct diagnosis [ 98 ]. Pediatric patients with high titers (over 10 times the cut-off) of anti-tTG antibodies, detectable EmA, HLA-DQ2/HLA-DQ8 positivity, and signs/symptoms suggestive of CD may skip duodenal biopsy as recommended by recent guidelines by the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) [ 99 ]. Although a large multicenter European study showed diagnostic accuracy of ESPGHAN criteria in identifying CD in children [ 100 ], it should be pointed out that these criteria are not followed worldwide. In fact, in some countries such as the USA, ESPGHAN criteria are not recommended because of the poor reproducibility of the anti-tTG assays [ 101 ]. Both advantages and disadvantages exist to biopsy for children with suspected celiac disease; however, most pediatric cases, especially those with low to medium anti-tTG2 titers, require histopathological assessment to confirm celiac disease diagnosis. In a recent study, Fuchs et al. [ 102 ] showed that the combination of anti-tTG (over 10 times the cut-off), EmA, and HLA-DQ2/HLA-DQ8 positivity (triple criteria) had a good accuracy across the range of pre-test probabilities in detecting adult patients with CD. Nonetheless, duodenal biopsy still represents a pillar in the diagnosis of adult patients with suspected CD.
Current standard of care is based on the “ four out of five rule ” [ 103 ], which indicates that four out of five of the following criteria are enough to establish CD diagnosis: (1) typical signs and symptoms (diarrhea and malabsorption); (2) antibody positivity; (3) HLA-DQ2 and/or HLA-DQ8 positivity; (4) intestinal damage (i.e., villous atrophy and minor lesions); and (5) clinical response to GFD. Additionally, this rule helps physicians to identify the various subtypes of CD, i.e., seronegative CD (absence of point 2), potential CD (absence of point 4), non-classic CD (absence of point 1), and non-responsive CD (absence of point 5).
Hematology and blood biochemistry tests
Routine blood tests can lead to suspect CD [ 104 ]. Low serum levels of hemoglobin, albumin, calcium, potassium, magnesium, and phosphorus are more commonly detected in CD with a classic rather than non-classic phenotype. Most patients develop an iron deficiency microcytic anemia with low ferritin values. Normocytic, macrocytic, or dimorphic anemia is less common in CD patients with an increased variability in the size of red blood cells due to concomitant malabsorption of folate and/or vitamin B12, particularly in cases associated with autoimmune atrophic gastritis [ 73 ]. Elevated levels of bone-specific alkaline phosphatase and a significant vitamin D3 deficiency can be found in patients with CD and osteopenia/osteoporosis [ 105 ]. A cryptogenic increase of transaminases may herald the presentation of CD even in the absence of other relevant symptoms. Notably, transaminases revert to normal within 6–12 months of a GFD [ 76 ]. In a moderate percentage of adult CD patients, a blood smear can detect changes in the membrane and cytoplasm of red blood cells (i.e., Howell–Jolly bodies), whereas pitted red cells can be identified by Nomarski phase contrast microscopy; both these red blood cell abnormalities suggest an underlying hyposplenism [ 106 ]. Another sign of hyposplenism is the detection of a marked thrombocytosis in association with a small (in the most severe cases even undetectable) spleen revealed by ultrasound. Macroscopically evident or even functional (no major changes at imaging) hyposplenism is a predisposing factor for the development of infectious diseases due to encapsulated bacteria (e.g., Pneumococcus, Meningococcus), and is associated with autoimmune diseases and complications such as refractory CD, ulcerative jejunoileitis, and lymphoma [ 107 , 108 ].
Over the last 20 years, the routine use of serological tests led to a significant increase in CD diagnoses. CD-related antibodies can identify subjects with suspected CD, further confirmed by histological evaluation [ 98 ]. In the early 1980s, anti-gliadin antibodies were the first serological marker used to screen patients at risk for CD. However, due to their low specificity, this serological test has been dismissed and its role is now confined to the possible identification of a subset of cases with non-celiac gluten/wheat sensitivity [ 109 ]. Currently, the serological diagnosis of CD is based on tests that are highly predictive and widely validated, including EmA, anti-tTG, and DGP [ 97 ]. CD-related antibodies belong to IgA and IgG classes, but only those of IgA class can be regarded as highly sensitive and specific for CD [ 97 ]. The use of IgG markers (except for DGP) is often misleading due to the high percentage of false positives, and their use should be limited to patients with IgA deficiency [ 110 ]. EmA is the antibody test with the highest diagnostic accuracy since it offers an absolute specificity if tested in third-level laboratories by expert operators [ 111 , 112 ]. The sensitivity of anti-tTG IgA is higher than that of EmA IgA (97% vs. 94%), while the specificity of tTG IgA is certainly lower than that of EmA (91 and 99%, respectively) (Table 3 ) [ 96 ]. False positives for anti-tTG normally display a low antibody titer (less than twice the cut off). A transient positivity for anti-tTG IgA, not associated with duodenal mucosal damage, has been reported in patients with type 1 diabetes at onset followed by a subsequent disappearance of antibodies within 6 months of their identification [ 113 ].
Another serological marker for CD is represented by DGP [ 96 ]. Compared to native peptides, the deamidation of gliadin by tTG makes the modified gliadin peptides more immunogenic. Initial studies reported an elevated sensitivity and specificity for CD [ 96 ], although other data showed a decrease in diagnostic accuracy [ 114 ]. IgG DGP are particularly useful in identifying CD in early childhood (age < 2 years) [ 115 ]. IgA DGP have been shown to be of little usefulness in diagnosing CD and therefore are not recommended for diagnosis [ 97 ]. In adult CD, serology should include testing anti-tTG IgA along with total IgA. Should anti-tTG IgA be positive at a high titer with normal total IgA level, a duodenal biopsy can be performed without assessing EmA. With a low titer anti-tTG IgA, EmA IgA testing is necessary and, if positive, a duodenal biopsy should be recommended to confirm CD diagnosis (Fig. 4 ).
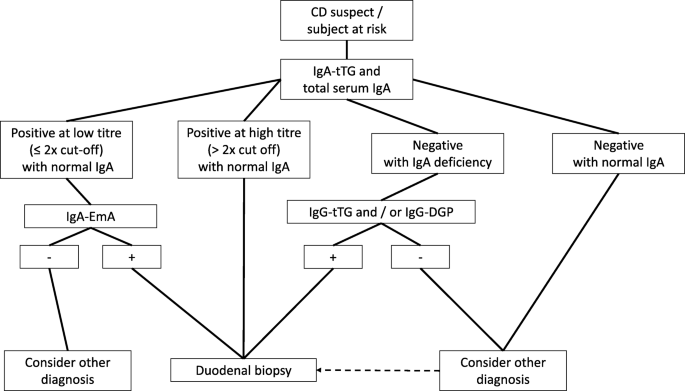
Diagnostic algorithm for celiac disease diagnosis
Strict compliance with a GFD in most CD patients leads to the disappearance or significant decrease of antibodies within 12 months (18–24 months if the antibody titer is very high) together with regrowth of the intestinal villi. IgA anti-tTG antibodies are the most commonly used test to monitor CD patients during follow-up, although their disappearance does not reflect the regrowth of intestinal villi [ 97 , 116 ]. Recent data from Choung et al. [ 117 ] demonstrated a very high specificity and sensibility of a new assay directed to identify the serum immune response to epitopes of the tTG-DGP complex. In addition to diagnosis, such markers can be useful for follow-up purposes, although further studies are eagerly needed. While waiting for the validation of a tTG–DGP complex assay, current serology is not enough for evaluating the response to GFD and the regrowth of villi [ 118 , 119 ].
Duodenal biopsy
Morphological evaluation of the duodenal biopsy is still of critical importance for confirming CD diagnosis. Histology remains the ‘gold standard’ for CD diagnosis [ 94 ]. In recent years, however, the histological criteria for CD have radically changed with the inclusion of mild villous atrophy and minimal lesions (characterized by an isolated increase in IELs) as possible expression of gluten-related intestinal damage [ 120 , 121 ]. Current recommendations are for four biopsies on the second duodenal portion and two biopsies at the bulb [ 122 ]. A fundamental principle for the correct evaluation is the orientation of biopsy samples using cellulose acetate Millipore filters [ 123 , 124 ]. The different types of CD-related lesions of the intestinal mucosa can be categorized into five stages according to the Marsh classification, modified by Oberhüber, which is currently used in all reference centers for the diagnosis of CD [ 120 ]. Type 1 and type 2 lesions, characterized by an increase in IELs (with or without crypt hyperplasia) and normal villi, compatible with, but non-specific for CD. Together with positive anti-tTG and EmA, minimal intestinal lesions indicate potential CD. In most cases, minimal lesions are attributable to other causes, including food allergies (e.g., cow milk proteins), Crohn’s disease, lymphocytic colitis, bacterial and parasitic intestinal infections, such as Giardia , common variable immunodeficiency, small intestinal bacterial overgrowth, non-steroidal anti-inflammatory drugs, and Helicobacter pylori infection (Box 1) [ 125 , 126 , 127 ].
In recent years, there has been a worrying increase in the number of diagnoses of CD incorrectly based on minimal lesions with no genetic and serological markers [ 128 ]. The IEL cytometric pattern is more accurate than subepithelial deposits of anti-TG2 IgA for identifying CD in lymphocytic enteritis [ 129 ]. The normal IEL cut-off has been established to be ≥25 lymphocytes over 100 epithelial cells. Even if it is well established that coeliac patients always display IEL counts ≥25%, a recent paper stressed the importance of a high IEL count for CD diagnosis underlining that the mean IEL count in untreated CD was 54 ± 18/100 enterocytes, whereas in non-CD patients the value was 13 ± 8 [ 130 ]. The typical lesion of CD shows villous atrophy with a change in the villi-to-crypt ratio (< 3:1 to 1:1) and an increase in IEL. This lesion, defined as type 3 in the Marsh–Oberhüber classification, is in turn subdivided into three stages depending on the severity of the atrophy, namely mild (3a), partial (3b), and subtotal atrophy (3c) [ 120 ]. Recently, Marsh et al. [ 131 , 132 ] argued against Oberhüber’s lesion III sub-division, claiming that splitting intestinal atrophy in three stages can be clinically irrelevant and sometimes misleading. In line with this theory no significant difference in IEL count was observed in mild, partial, and subtotal villous atrophy [ 130 ]. In an attempt to simplify the histopathological grading and therefore the relationship between pathologists and clinicians, Corazza and Villanacci proposed a classification from five to three stages [ 121 ]. Notably, the lesions that characterize CD were divided into two categories – non-atrophic (grade A) and atrophic (grade B) – with the latter being further subcategorized into B1, in which the villi-to-crypt ratio is less than 3:1 (with identifiable villi), and B2, in which villi are entirely atrophic. Grade A lesions, characterized by a pathological increase in the number of IELs, better identified by immunohistochemical staining for CD3, include type 1 and 2 lesions based on the Marsh–Oberhüber classification; grade B1 lesions include the 3a and 3b lesions, while grade B2 corresponds to 3c (Fig. 5 ) [ 121 ]. In some patients with more distal disease or in those with contraindication to biopsy, videocapsule endoscopy can be recommended [ 133 ].
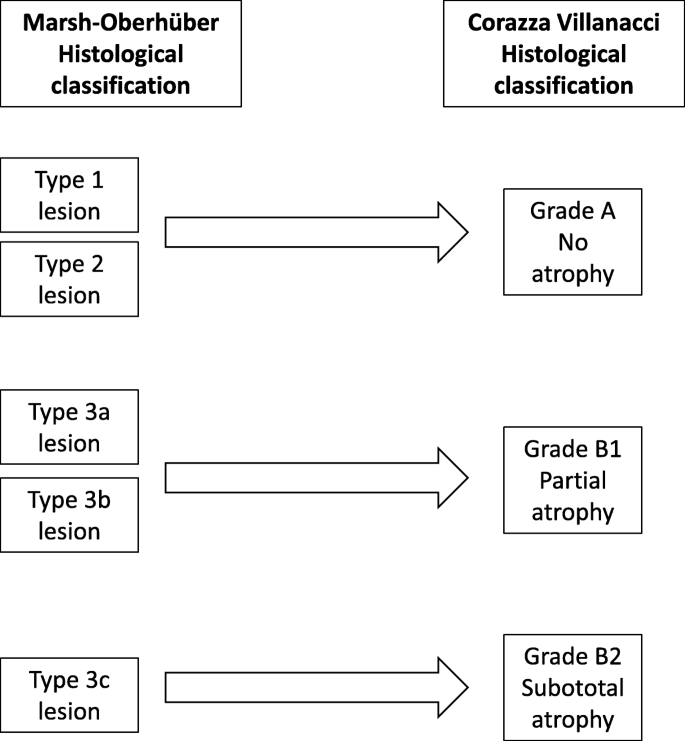
Comparison between the two classifications for the duodenal biopsy
Classification of variants of CD
Potential cd.
In recent years, an increasing number of patients have antibody positivity (IgA EmA and anti-tTG) for CD with HLA-DQ2/HLA-DQ8 and lack of villous atrophy [ 134 , 135 ]. For this category of patients, which represents around 10% of subjects with CD, the term potential celiac disease has been adopted [ 69 ]. In patients with potential CD the intestinal mucosa may be normal (Marsh 0) or slightly inflamed (increased number of IELs, i.e., Marsh 1) [ 135 ]. Despite the absence of severe lesions in the intestinal mucosa, these patients may have GI and/or extraintestinal symptoms or be entirely asymptomatic [ 2 , 135 ]. Although the criteria for diagnosing this condition are clear, potential CD still remains a poorly studied area, with many unsettled questions and contrasting results in the studies conducted so far [ 135 , 136 , 137 , 138 , 139 , 140 , 141 ]. In children, over 80% of patients with potential CD are asymptomatic and the remaining 20% more commonly experience intestinal symptoms such as malabsorption, chronic diarrhea, and recurrent abdominal pain rather than extraintestinal signs such as iron-deficiency anemia, hypertransaminasemia, and short stature [ 137 , 138 , 141 ]. In adults, however, several studies have shown that the symptomatic phenotype in subjects with potential CD is much more common than in children, and it is primarily characterized by extraintestinal symptoms [ 135 , 136 , 139 , 140 ]. One controversial issue concerns whether subjects with potential CD should be treated by a GFD. The actual evidence suggests that a GFD should be recommended only to subjects with symptomatic potential CD. On the other hand, patients with asymptomatic potential CD are allowed to continue a gluten-containing diet while being followed-up with close clinical, serological, and histological control visits (in our experience every 6 months) [ 135 , 136 , 137 , 138 , 139 , 140 ]. Studies have reported possible fluctuation with spontaneous normalization of serological markers in patients with potential CD left on a gluten-containing diet. Few patients with potential CD consuming a gluten-containing diet develop full-blown villous atrophy [ 135 , 137 , 138 , 140 , 142 ]. In our study, only 6% of these subjects developed villous atrophy over a mean follow-up period of 3 years, whereas symptomatic subjects should be treated as they show a clear clinical improvement in symptoms with a GFD [ 135 ].
Seronegative CD
Although the specific antibodies for CD can be detected in the vast majority of patients, a small number of CD patients (around 2–3%) test negative for serological markers. In these cases, the diagnosis is closely connected to the detection of villous atrophy on the duodenal histology [ 94 , 139 , 143 ]. Performing a genetic test for CD remains a fundamental step since its negative result definitively rules out the disease and prompts physicians to seek for other causes of villous atrophy. A seronegative CD can be confirmed 1 year after the beginning of a GFD, a convenient time to demonstrate an improvement in both symptoms and histology. The diagnostic complexity of this particular variant of CD is due to the differential diagnosis with other conditions involving villous atrophy, such as parasitic infections ( Giardia lamblia ), autoimmune enteropathy, bacterial contamination of the small intestine, common variable immunodeficiency, eosinophilic gastroenteritis, drug-induced enteropathy (angiotensin II receptor antagonists, i.e., olmesartan and other sartans, non-steroidal anti-inflammatory drugs, and mycophenolate), intestinal lymphoma, Crohn’s disease, tropical sprue, HIV enteropathy, and Whipple disease (Fig. 6 ) [ 94 , 144 , 145 ]. Of all villous atrophies lacking CD antibodies, 28–45% are due to an underlying seronegative CD [ 94 , 146 , 147 ]. Seronegative CD patients display a classic clinical phenotype, characterized by diarrhea and malabsorption, a clear female gender prevalence, and have a higher risk of morbidity and mortality compared with antibody-positive CD patients [ 94 , 147 ]. Furthermore, compared to classic CD, seronegative patients have a greater association with autoimmune diseases and a higher risk of developing refractory disease. This increased morbidity could be partly due to the late diagnosis of this condition, which on average is around 50 years of age [ 94 ].
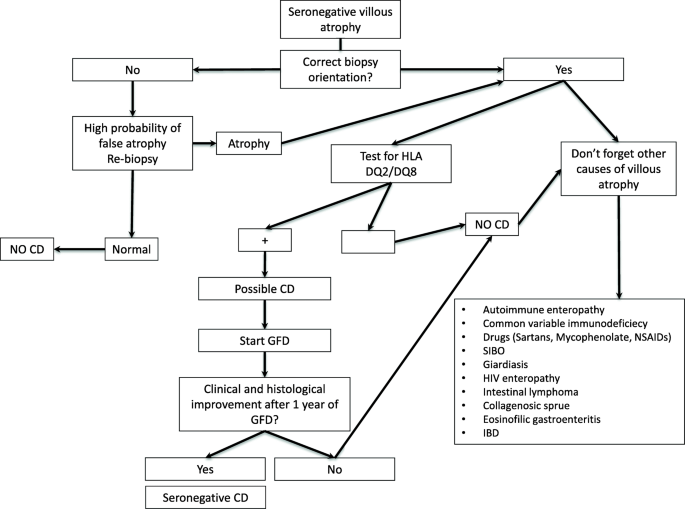
Diagnostic algorithm for seronegative villous atrophy. SIBO small intestinal bacterial overgrowth
Assessment of ongoing signs and symptoms in CD
The majority of the patients with CD exhibit a symptomatic and mucosal response to the GFD. Some patients, however, fail to have complete control of symptoms and normalization of villous structure despite attempted adherence to the GFD. These patients have traditionally been referred to as non-responsive CD [ 95 , 148 ]; however, this terminology has resulted in confusion as, in many cases, manifestations are due to associated conditions rather than CD. In light of both emerging tests for CD monitoring, such as GIPs, and emerging novel therapies for active CD, we propose updating this classification (formerly non-responsive CD). When evaluating a patient with CD on a GFD and with ongoing signs or symptoms, the initial step is the differentiation between ongoing active CD (OACD) and the presence of associated CD conditions (ACDCs). OACD can be seen in three scenarios – (1) slow response, where there is progressive improvement in symptoms and mucosal damage, but full remission does not occur for at least 1–2 years; (2) RCD, where there is ongoing severe enteropathy and malabsorptive symptoms after 6–12 months on a GFD; and (3) gluten exposure, where, despite adequate understanding of the GFD and attempted adherence, gluten avoidance is insufficient to result in symptomatic or histologic remission. This is the most frequent cause of OACD and can be due to very high sensitivity to a low level of gluten exposure or an inability of the patient to achieve standard recommended gluten restriction. Conversely, when patients with ongoing symptoms are found not to have OACD, generally when small bowel assessment shows minimal ongoing enteropathy and significant gluten exposure is excluded, investigation of possible ACDCs is recommended. ACDCs include IBS, small intestinal bacterial overgrowth, microscopic colitis, lactose intolerance, fructose intolerance, diverticular disease, Crohn’s disease, pancreatic insufficiency, and autoimmune and drug-induced enteropathy, and should be evaluated according to clinical suspicion (Fig. 3 ) [ 95 , 148 ].
CD complications
It has been widely shown that a late diagnosis of CD (after the age of 50) and/or not following a strict GFD can lead to a higher mortality compared to that of the general population [ 149 ]. Although rare (around 1% of patients diagnosed with CD) [ 150 ], the complications of CD include hyposplenism, RCD, intestinal lymphoma, small bowel adenocarcinoma, and ulcerative jejunoileitis. Complications should be suspected in all patients who, despite adherence to a GFD, complain of an unexplained persistence or re-exacerbation of symptoms (i.e., diarrhea, intestinal sub-occlusion, abdominal pain, weight loss, fever, and severe asthenia). These complications occur more commonly when a diagnosis of CD was established in elderly patients and/or in those who are homozygous for DQ2 not observing a strict GFD [ 151 ].
Hyposplenism
Anatomical or functional hyposplenism can be identified in around 30% of adult patients with CD, with prevalence increasing up to 80% in patients with complications [ 107 , 152 ]. In CD cases, the detection of a small-size spleen on abdominal ultrasound should guide physicians to confirm functional hyposplenism by evaluating Howell–Jolly bodies (on a peripheral blood smear) or pitted red cells with phase-contrast microscopy (see above) [ 107 , 152 ]. Splenic hypofunction is closely associated not only with the development of complications and other autoimmune diseases associated with CD but also encapsulated bacterial infections (i.e., Pneumococcus , Haemophilus influenzae , Meningococcus ) [ 107 ]. Because of the greater risk of developing infections (in some cases lethal or with severe sequelae) from encapsulated bacteria, anti-pneumococcal and anti-meningococcal vaccinations are recommended in this subgroup of patients [ 106 , 107 , 152 ].
Refractory CD
RCD represents about 10% of all OACD cases [ 148 ] and approximately 1–1.5% of total cases of CD [ 153 ]. This condition is characterized by symptoms of malabsorption, weight loss, and diarrhea associated with persistent villous atrophy after at least 1 year on a strict GFD, confirmed by negative CD serology [ 69 ]. Before thinking of RCD, physicians should rule out other more frequent causes of ongoing signs and symptoms of CD, as previously reported [ 95 , 148 ]. Refractory CD is in turn subdivided into two categories, primary and secondary, depending on whether the patients had a symptomatic response since the beginning of GFD, or they had a recurrence of symptoms after a more or less long period of improvement.
There are two subtypes of RCD – type 1, where the IEL population has a normal CD3 + CD8 + phenotype, and type 2, with a clonal presentation of surface CD3 − /intracytoplasmic CD3 + IELs along with monoclonal rearrangement of the gamma-chain of the T cell receptor [ 153 ]. This distinction into two subtypes is fundamental for therapeutic management and prognosis; in fact, type 2 displays a 5-year mortality rate of 55% vs. 7% for type 1 [ 154 ]. The mortality of patients with type 2 RCD is primarily due to the development of intestinal lymphoma, which appears to occur more often in male patients, although CD is more commonly detectable in female patients (female-to-male ratio 3:1) [ 155 ]. A diagnosis of RCD should always be suspected by persistent villous atrophy despite a strict, 1-year GFD, negative serology (some cases may show the persistence of low-titer CD-related antibodies), the exclusion of other causes of persistent villous atrophy, and phenotyping of the intestinal lymphocytic population aimed to confirm the presence (type 2) or absence (type 1) of a monoclonal rearrangement of T cell receptor. In all cases of type 2 RCD, it is essential to perform, at diagnosis, a computed tomography (CT) and/or magnetic resonance (MR) enterography followed by positron emission tomography (PET), capsule endoscopy, and enteroscopy in order to rule out the progression to intestinal lymphoma [ 152 , 154 ]. Due to this risk, in subjects with a diagnosis of type 2 RCD, a capsule endoscopy has been recommended once a year at the follow-up [ 156 ]. From a therapeutic perspective, the management of type 1 RCD is based on immunosuppressive therapy containing steroids, azathioprine, 6-mercaptopurine, and methotrexate, whereas type 2 therapy is based on additional medications, including cyclosporine and chemotherapy such as cladribine and fludarabine associated with anti-CD52 monoclonal antibodies (alemtuzumab). Promising results have been recently reported by treating patients with anti-IL-15 antibodies (AMG-714). In certain cases, an autologous stem cell transplantation has been attempted with promising results [ 154 , 155 , 156 ].
Intestinal lymphoma
The association between CD and cancers has been known for over 50 years [ 157 ] and a delayed diagnosis of CD exposes patients to an increased risk of developing neoplastic diseases [ 158 ]. In recent years, several studies have reported a growing incidence from 6 to 9 times higher than that of the general population for non-Hodgkin T cell intestinal lymphoma and, to a lesser extent, also B cell lymphoma [ 158 ]. In most cases, the development of intestinal lymphoma is preceded by type 2 RCD that develops into malignant disease in 33–52% of cases within 5 years from diagnosis. More rarely, intestinal lymphoma may develop from type 1 RCD, with a rate of 14% over 5 years [ 159 ]. Treatment in cases of CD-related intestinal lymphoma involves chemotherapy, i.e., high-dose ifosfamide, epirubicin, and etoposide methotrexate, followed by autologous stem cell transplantation. If lymphoma includes an elevated expression of CD30 (> 80% of the neoplasm) it is possible to use biologic therapy with anti-CD30 associated with monomethyl auristatin E (brentuximab vedotin) and a chemotherapy regimen containing cyclophosphamide–doxorubicin–prednisone followed by autologous stem cell transplantation [ 159 ]. Recent data indicate that NKp46, a NK receptor expressed by lymphocytes, can be a biomarker as well as a possible therapeutic target for T cell lymphoproliferative diseases, i.e., type 2 RCD and enteropathy-associated T cell lymphoma [ 160 ].
Small bowel adenocarcinoma
Small bowel adenocarcinoma is an extremely rare cancer in the general population (5.7 cases/1,000,000 people per year) but it is much more common in patients with CD (odds ratio reported in the literature ranges between 4.3 to 60.0), usually being detectable in the jejunum [ 161 ]. Compared to lymphomas, small bowel adenocarcinoma is rare, although increasingly detectable in the clinic. Nowadays, however, the diagnosis of this cancer occurs together with CD. Unlike intestinal lymphoma, the small bowel adenocarcinoma is not preceded by RCD and occurs more commonly in female patients [ 150 ]. The onset of a sudden intestinal (sub)/occlusion and/or anemia, particularly in patients with a late diagnosis of CD and patients who have been following a GFD for a short period of time, are clinical features suggestive of an underlying small bowel adenocarcinoma. A thorough diagnostic work-up is mandatory and requires a wide array of imaging tests (e.g., CT/MR-enterography, PET, capsule endoscopy, and enteroscopy) [ 162 ].
Follow-up for CD in adults
A well-defined follow-up strategy should be agreed by physicians and patients once CD has been diagnosed. Usually, the first follow-up visit is planned within 6 months from diagnosis and then every 12–24 months (every 3–6 months if complications occur) is adequate to confirm compliance with the GFD, rule out the onset of autoimmune diseases and metabolic changes, and, most importantly, to allow for the early diagnosis of any complications [ 163 ]. Patients should undergo a consultation with a dietician and follow-up blood tests including complete blood count, anti-tTG IgA (or IgG in case of IgA deficiency), thyroid stimulating hormone, anti-thyroidperoxidase, anti-thyroglobulin, ferritin, folate, vitamin D3, transaminases, and a metabolic profile [ 163 ]. The first follow-up should include a screening of antinuclear antibodies and non-organ-specific autoantibodies in order to rule out the presence of markers predictive of autoimmune diseases associated with CD. Should the antinuclear antibodies test reveal a high titer along with extractable nuclear antigen antibody positivity, this information might be useful to investigate for other autoimmune CD-associated disorders, e.g., primary biliary cholangitis and Sjogren syndrome [ 2 ]. In adults, a bone density scan should be performed after 12–18 months of a GFD and repeated regularly only if abnormal or in case of other indications. Subjects with osteopenia should be treated with supplements containing calcium and vitamin D, while possible treatment with bisphosphonates should be considered in cases of osteoporosis. Body weight increase may occur as a consequence of an excessive consumption of dietary products high in vegetable fats (colza, palm, and coconut oil) commonly present in GFD [ 164 ]. Therefore, nutritional counselling is advisable to prevent metabolic complications, including liver steatosis, during follow-up. On the other hand, patients who are starting GFD should be tested with an abdominal ultrasound to exclude spleen abnormality (i.e., hyposplenism) [ 165 ].
Notwithstanding a strict GFD, CD patients may experience abdominal symptoms ascribable to IBS in 30–50% of cases; these symptoms may respond to dietary recommendations (e.g., reduction of insoluble fiber intake or fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) as well as symptomatic drug therapy [ 166 ].
A self-adapted GFD, without the support of a nutritionist, can cause vitamin and trace metal deficiency, which should be supplemented if needed, particularly when patients report the onset of asthenia [ 167 ]. Additionally, constipation, which can be associated with a GFD, requires appropriate management based on non-irritant (e.g., osmotic) laxatives [ 168 ].
Should a CD-related complication occur, follow-up visits should be more frequent, i.e., every 3–6 months [ 156 ]. In these circumstances, in addition to standard tests (as previously listed), protein electrophoresis, lactate dehydrogenase, and beta-2 microglobulin testing should be included. Upper endoscopy should be performed (with new duodenal biopsies) along with abdominal ultrasound, as well as CT/MR-enterography, PET, capsule endoscopy, and enteroscopy [ 154 , 155 , 156 ].
Physicians may consider (even if not recommended by current guidelines) performing a follow-up duodenal biopsy in adults in order to check the regrowth of villi in patients on a GFD, keeping in mind that the average time to the restitutio ad integrum of the villi could take up to 3 years. A second duodenal biopsy after GFD should be recommended only in those patients with persisting symptoms and demonstrable laboratory deficiencies of micronutrients [ 133 ].
Finally, GIP assessment, a controversial test still awaiting further validation, can be performed on stool samples and may be useful for monitoring the adherence to a GFD [ 48 ].
Follow-up for CD in children
Currently, the follow-up of CD in children is lacking standardized evidence-based recommendations [ 169 ]. Children with CD should be followed up after 6 months from diagnosis and then every year in order to check symptomatic improvement, adherence to GFD, quality of life, and progressive normalization of CD-related antibodies. Laboratory tests and biochemical evaluation is crucial in these patients and should be tailored on case-by-case basis. As for adults, autoimmune thyroiditis should always be screened. Duodenal biopsy monitoring is unnecessary after a GFD has been instituted. However, should the patient have no or partial clinical response to gluten withdrawal, a careful assessment should be recommended to rule out inadvertent gluten ingestion or poor adherence to a GFD. Furthermore, in this subset of poorly responsive patients, a duodenal histopathology is advisable [ 119 , 169 ]. At variance to adults, children hardly ever develop complications, indeed only a few case reports of refractory CD have been reported [ 170 ].
Diet and new treatments
Currently, the only effective treatment available for CD is a strict GFD for life since it leads to the resolution of intestinal and extraintestinal symptoms, negativity of autoantibodies, and the regrowth of the intestinal villi. In addition, the diet offers a partial protective effect towards several complications. However, these crucial advantages are accompanied by some disadvantages, including a negative impact on quality of life, psychological problems, fear of involuntary/inadvertent contamination with gluten (as demonstrated in multicenter GIP studies) [ 48 ], possible vitamin and mineral deficiencies, metabolic syndrome, an increased cardiovascular risk, and often severe constipation [ 171 , 172 , 173 ]. Most of these CD-related drawbacks can be overcome by instructing the patient about the risks of an uncontrolled gluten-free regimen and by providing nutritional recommendations by a dietician with experience in CD. From a psychological perspective, the support a psychologist could be highly useful in accepting the disease [ 174 ].
Due to the relevant burden induced by gluten withdrawal with consequent worsening of quality of life, about 40% of CD patients are unsatisfied with their alimentary regimen and they would be keen to explore alternative treatments [ 175 ]. In recent years, researchers have attempted to meet the requests of CD patients seeking therapies different from diet [ 176 ]. Clinical trials are currently in progress, but only few have reached later clinical trial phases, namely those with larazotide acetate and gluten-specific proteases from a bacterial mix (ALV003) [ 177 , 178 , 179 , 180 ]. Larazotide acetate is a zonulin antagonist blocking tight junction disassembly, thereby limiting gluten crossing a permeable intestinal mucosal barrier [ 177 ]. Larazotide has shown efficacy in gluten-related symptom control rather than in restoring complete epithelial barrier integrity and preventing gluten from crossing the mucosal lining [ 177 ]. Taken together, the data so far published indicate that larazotide may be beneficial in allowing patients to tolerate minimal amounts of gluten such as those derived from inadvertent ingestion or probably for ‘gluten-free holidays’, i.e., a short period during which patients are allowed to eat a minimal amount of gluten. ALV003 targets gluten and degrades it into small fragments in the stomach before they pass into the duodenum [ 178 ]. This strategy has also been demonstrated to be able to ‘digest’ only small quantities of gluten and thus would be effective against contamination but not to protect patients from the effects driven by large quantities of gluten [ 178 ]. However, a recent phase 2b study by Murray et al. [ 180 ] showed that ALV003 (or latiglutenase) did not improve histologic and symptoms scores in 494 CD patients with moderate to severe symptoms versus placebo. IL-15 monoclonal antibodies (AMG 714) are being investigated in phase 2 studies in both gluten challenge and RCD type II patients, but additional safety studies are needed for the acquisition and competition of the license. Finally, vaccination (Nexvax2) is another possible therapeutic strategy aimed at desensitizing patients with CD to gliadin peptides. Although abdominal pain and vomiting were major side effects, the trial passed phase 1. Vaccines could represent a definitive cure for CD should data show actual efficacy [ 181 ].
Can CD be prevented?
Several retrospective studies have suggested that breastfeeding, modality of delivery, and time of gluten introduction in the diet of infants at risk for CD may affect the incidence of the disease. However, the data supporting the role of these factors in the risk of developing CD is limited by their retrospective design and have been criticized by alternative interpretations [ 182 , 183 , 184 ]. Two recent landmark studies [ 19 , 21 ], which prospectively screened infants with a first-degree family member with CD from birth, found that CD develops quite early in life in this risk group, demonstrating that early environmental factors may be crucial in the development of CD. However, these studies failed to identify possible targets to prevent CD, leading to the gut microbiota as the key element to scrutinize for possible innovative preventive strategies. In this line, viral (e.g., rotavirus) GI infections may potentiate subsequent development of CD. Thus, rotavirus vaccination seems to significantly decrease the risk of CD, in particular among children with early (before 6 months of age) gluten exposure [ 185 ]. The ongoing Celiac Disease Genomic, Environment, Microbiome, and Metabolomic study has been designed to identify potential primary prevention targets by establishing microbiome, metabolomic, and/or environmental factors responsible for loss of gluten tolerance, thus switching genetic predisposition to clinical outcome [ 186 ].
Although there has been a substantial increase in the number of CD diagnoses over the last 30 years, many patients remain undiagnosed [ 187 ]. The flow-chart for identifying CD in adults must always include both serology and intestinal biopsy, whereas genetics should be performed only in selected cases. Diagnostic criteria should help physicians in avoiding misdiagnosis and missing cases of CD (i.e., seronegative patients with classic symptoms not undergoing biopsy) and preserve people from an unjustified GFD. The treatment for CD is still primarily a GFD, which requires significant patient education, motivation, and follow-up. Slow response occurs frequently, particularly in people diagnosed in adulthood. Persistent or recurring symptoms should lead to a review of the patient’s original diagnosis, exclude alternative diagnoses, evaluation of GFD quality, and serologic testing as well as histological assessment in order to monitor disease activity. In addition, evaluation for disorders that could cause persistent symptoms and complications of CD, such as refractory CD or lymphoma, should be pursued. The future opens to new therapeutic and preventive strategies, which are expected to improve the patient’s quality of life and pave the way to a definitive cure for this old disease.
Box 1 Causes for the increased number of intraepithelial lymphocytes in the intestinal mucosa with normal villous architecture
Potential celiac disease
Non-celiac gluten sensitivity
Food allergies (cereals, milk proteins, soy derivatives, fish, rice, chicken)
Infectious (viral enteritis, Giardia, Cryptosporidium, Helicobacter pylori )
Bacterial contamination of the small intestine
Drugs (e.g., non-steroidal anti-inflammatory drugs)
Immune system diseases (Hashimoto’s thyroiditis, rheumatoid arthritis, systemic erythematosus lupus, type 1 diabetes mellitus, autoimmune enteropathy)
Common variable immune deficiency
Chronic inflammatory intestinal diseases (Crohn’s disease, ulcerative colitis)
Lymphocytic colitis
Availability of data and materials
Abbreviations.
Associated celiac disease conditions
Celiac disease
Computed tomography
Deamidated gliadin peptides antibodies
Anti-endomysial antibodies
European Society for Paediatric Gastroenterology Hepatology and Nutrition
- Gluten-free diet
Gastrointestinal
Gluten immunogenic peptides
Human leukocyte antigen
Irritable bowel syndrome
Intraepithelial lymphocytes
Interleukin
Magnetic resonance
Natural killer
Ongoing active celiac disease
Positron emission tomography
Refractory celiac disease
Transglutaminase 2
tissue transglutaminase
Fasano A, Catassi C. Celiac disease. N Engl J Med. 2012;367:2419–26.
Article CAS PubMed Google Scholar
Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194.
Article PubMed PubMed Central Google Scholar
Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. 2013;10:383–92.
Article CAS PubMed PubMed Central Google Scholar
de Lorgeril M, Salen P. Gluten and wheat intolerance today: are modern wheat strains involved? Int J Food Sci Nutr. 2014;65:577–81.
Article PubMed CAS Google Scholar
van den Broeck HC, de Jong HC, Salentijn EM, et al. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac disease. Theor Appl Genet. 2010;121:1527–39.
Article PubMed PubMed Central CAS Google Scholar
Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18:105–20.
Corazza GR, Andreani ML, Biagi F, et al. The smaller size of the ‘coeliac iceberg’ in adults. Scand J Gastroenterol. 1997;32:917–9.
Ivarsson A, Persson LA, Juto P, et al. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63–8.
Riestra S, Fernandez E, Rodrigo L, et al. Prevalence of coeliac disease in the general population of northern Spain. Scand J Gastroenterol. 2000;35:398–402.
Volta U, Bellentani S, Bianchi FB, et al. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46:1500–5.
Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–95.
Article PubMed Google Scholar
Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–44.
Singh P, Arora S, Singh A, et al. Prevalence of celiac disease in Asia: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;3:1095–101.
Article Google Scholar
Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42:530–8.
Hall EJ, Batt RM. Dietary modulation of gluten sensitivity in a naturally occurring enteropathy of Irish setter dogs. Gut. 1992;33:198–205.
Okada H, Kuhn C, Feillet H, Bach J. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
Verdu EF, Galipeau HJ, Jabri B. Novel players in celiac disease pathogenesis: the role of gut microbiota. Nat Rev Gastroenterol Hepatol. 2015;185:2969–82.
Google Scholar
Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12:507–15.
Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295–303.
Liu E, Lee HS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–9.
Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304–15.
Mazzilli MC, Ferrante P, Mariani P, et al. A study of Italian pediatric celiac disease patients confirms that the primary HLA association is to the DQ (α1 ∗ 0501, β1 ∗ 0201) heterodimer. Human Immunol. 1992;33:133–9.
Article CAS Google Scholar
Dieli-Crimi R, Cénit MC, Núñez C. The genetics of celiac disease: a comprehensive review of clinical implications. J Autoimmun. 2015;64:26–41.
Silano M, Vincentini O, De Vincenzi M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr Med Chem. 2009;16:1489–98.
Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9.
Jelinkova L, Tuckova L, Cinova J, et al. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004;571:81–5.
Lammers KM, Khandelwal S, Chaudhry F, et al. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology. 2011;132:432–40.
Picarelli A, Di Tola M, Sabbatella L, et al. 31–43 amino acid sequence of the alpha-gliadin induces anti-endomysial antibody production during in vitro challenge. Scand J Gastroenterol. 1999;34:1099–102.
Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–23.
Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–5.
Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113:4435–40.
CAS PubMed Google Scholar
Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–9.
Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106:16799–804.
El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–15.
Smecuol E, Sugai E, Niveloni S, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005;3:335–41.
Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9.
Simpson M, Mojibian M, Barriga K, et al. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:563–72.
Duerksen DR, Wilhelm-Boyles C, Veitch R, et al. A comparison of antibody testing, permeability testing, and zonulin levels with small-bowel biopsy in celiac disease patients on a gluten-free diet. Dig Dis Sci. 2010;55:1026–31.
Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–19.
Hollon J, Puppa EL, Greenwald B, et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7:1565–76.
Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66.
Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in celiac disease. J Immunol. 2006;176:2512–21.
Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204.
Monsuur AJ, de Bakker PI, Alizadeh BZ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–4.
Loeff T, Araya M, Pérez-Bravo F. Frequency of MYO9B polymorphisms in celiac patients and controls. Rev Esp Enferm Dig. 2012;104:566–71.
Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–7.
Schumann M, Richter JF, Wedell I, et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut. 2008;57:747–54.
Moreno ML, Cebolla Á, Muñoz-Suano A, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut. 2017;66:250–7.
Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–54.
Kim SM, Mayassi T, Jabri B. Innate immunity: actuating the gears of celiac disease pathogenesis. Best Pract Res Clin Gastroenterol. 2015;29:425–35.
Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–408.
Barone MV, Troncone R, Auricchio S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int J Mol Sci. 2014;15:20518–37.
Cinova J, Palova-Jelinkova L, Smythies LE, et al. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201–9.
Lammers KM, Chieppa M, Liu L, et al. Gliadin induces neutrophil migration via engagement of the formyl peptide receptor, FPR1. PLoS One. 2015;10:e0138338.
Stamnaes J, Sollid LM. Celiac disease: autoimmunity in response to food antigen. Semin Immunol. 2015;27:343–52.
Tang F, Chen Z, Ciszewski C, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J Exp Med. 2009;206:707–19.
Tjon JM, van Bergen J, Koning F. Celiac disease: how complicated can it get? Immunogenetics. 2010;62:641–51.
Pagliari D, Urgesi R, Frosali S, et al. The interaction among microbiota, immunity, and genetic and dietary factors is the condicio sine qua non celiac disease can develop. J Immunol Res. 2015;2015:123653.
CAS PubMed PubMed Central Google Scholar
Hüe S, Mention JJ, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77.
Senger S, Sapone A, Fiorentino MR, et al. Celiac disease histopathology recapitulates hedgehog downregulation, consistent with wound healing processes activation. PLoS One. 2015;10:e0144634.
Ju JM, Marietta EV, Murray JA. Generating transgenic mouse models for studying celiac disease. Methods Mol Biol. 2015;1326:23–33.
Schumann M, Siegmund B, Schulzke JD, Fromm M. Celiac disease: role of the epithelial barrier. Cell Mol Gastroenterol Hepatol. 2017;3:150–62.
Olivares M, Benítez-Páez A, de Palma G, et al. Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: the PROFICEL study. Gut Microbes. 2018;9:551–8.
PubMed PubMed Central Google Scholar
Chander AM, Yadav H, Jain S, et al. Cross-talk between gluten, intestinal microbiota and intestinal mucosa in celiac disease: recent advances and basis of autoimmunity. Front Microbiol. 2018;9:2597.
Sellitto M, Bai G, Serena G, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One. 2012;7:e33387.
Olivares M, Neef A, Castillejo G, et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406–17.
Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and nutrition examination surveys from 1988 to 2012. Am J Gastroenterol. 2015;110:455–61.
Fasano A. Celiac disease: how to handle a clinical chamaleon. N Engl J Med. 2003;348:2568–70.
Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;6:43–52.
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318:647–56.
Vivas S, Ruiz de Morales JM, Fernandez M, et al. Age-related clinical, serological, and histopathological features of celiac disease. Am J Gastroenterol. 2008;103:2360–5.
Reilly NR, Aguilar K, Hassid BG, et al. Celiac disease in normal-weight and overweight children: clinical features and growth outcomes following a gluten-free diet. J Pediatr Gastroenterol Nutr. 2011;53:528–31.
PubMed Google Scholar
Baydoun A, Maakaron JE, Halawi H, et al. Hematological manifestations of celiac disease. Scand J Gastroenterol. 2012;47:1401–11.
Kamycheva E, Goto T, Camargo CA Jr. Celiac disease is associated with reduced bone mineral density and increased FRAX scores in the US National Health and nutrition examination survey. Osteoporos Int. 2017;28:781–90.
Krzywicka B, Herman K, Kowalczyk-Zając M, Pytrus T. Celiac disease and its impact on the oral health status – review of the literature. Adv Clin Exp Med. 2014;23:675–81.
Volta U, De Franceschi L, Lari F, et al. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352:26–9.
Volta U, Caio G, Tovoli F, De Giorgio R. Gut-liver axis: an immune link between celiac disease and primary biliary cirrhosis. Expert Rev Gastroenterol Hepatol. 2013;7:253–61.
Volta U, Tovoli F, Caio G. Clinical and immunological features of celiac disease in patients with type 1 diabetes mellitus. Expert Rev Gastroenterol Hepatol. 2011;5:479–87.
Caio G, De Giorgio R, Venturi A, et al. Clinical and immunological relevance of anti-neuronal antibodies in celiac disease with neurological manifestations. Gastroenterol Hepatol Bed Bench. 2015;8:146–52.
Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metanalysis. Am J Obstet Gynecol. 2016;214:225–34.
Farthing MJG, Edwards CRW, Rees LH, Dawson AM. Male gonadal function in coeliac disease: sexual dysfunction, infertility and semen quality. Gut. 1982;23:608–14.
Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561–71.
Gale L, Wimalaratna H, Brotodiharjo A, Duggan JM. Down’s syndrome is strongly associated with coeliac disease. Gut. 1997;40:492–6.
Bonamico M, Pasquino AM, Mariani P, et al. Prevalence and clinical picture of celiac disease in turner syndrome. J Clin Endocrinol Metab. 2002;87:5495–8.
Giannotti A, Tiberio G, Castro M, et al. Coeliac disease in Williams syndrome. J Med Genet. 2001;38:767–8.
Caio G, De Giorgio R, Ursini F, et al. Prevalence of celiac disease serological markers in a cohort of Italian rheumatological patients. Gastroenterol Hepatol Bed Bench. 2018;11:244–9.
Volta U, De Franceschi L, Molinaro N, et al. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci. 1998;43:2190–5.
Volta U, Rodrigo L, Granito A, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol. 2002;97:2609–13.
Volta U, Bardazzi F, Zauli D, et al. Serological screening for coeliac disease in vitiligo and alopecia areata. Br J Dermatol. 1997;136:801–2.
Oleary C, Walsh CH, Wieneke P, et al. Coeliac disease and autoimmune Addison’s disease: a clinical pitfall. QJM. 2002;95:79–82.
Cataldo F, Marino V, Ventura A, et al. Prevalence and clinical features of selective immunoglobulin a deficiency in coeliac disease: an Italian multicentre study. Gut. 1998;42:362–5.
Curione M, Barbato M, De Biase L, et al. Prevalence of coeliac disease in idiopathic dilated cardiomiopathy. Lancet. 1999;354:222–3.
Caio G, De Giorgio R, Volta U. Coeliac disease and dermatitis herpetiformis. Lancet. 2018;392:916–7.
Volta U, Caio G, Boschetti E, et al. Seronegative celiac disease: shedding light on an obscure clinical entity. Dig Liver Dis. 2016;48:1018–22.
Mooney PD, Evans KE, Singh S, Sanders DS. Treatment failure in coeliac disease: a practical guide to investigation and treatment of non-responsive and refractory coeliac disease. J Gastrointest Liver Dis. 2012;21:197–203.
Volta U, Granito A, Fiorini E, et al. Usefulness of antibodies to deamidated gliadin peptides in celiac disease diagnosis and follow-up. Dig Dis Sci. 2008;853:1582–8.
Volta U, Tovoli F, Piscaglia M, et al. Old and new serological test for celiac disease screening. Exp Rev Gatroenterol Hepatol. 2010;4:31–5.
Caio G, Volta U. Coeliac disease: changing diagnostic criteria? Gastroenterol Hepatol Bed Bench. 2012;5:119–22.
Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60.
Werkstetter KJ, Korponay-Szabó IR, Popp A, et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology. 2017;153:924–35.
Egner W, Shrimpton A, Sargur R, et al. ESPGHAN guidance on coeliac disease 2012: multiples of ULN for decision making do not harmonise assay performance across centres. J Pediatr Gastroenterol Nutr. 2012;55:733–5.
Fuchs V, Kurppa K, Huhtala H, et al. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment Pharmacol Ther. 2019;49:277–84.
Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691–3.
Kostopoulou O, Devereaux-Walsh C, Delaney BC. Missing celiac disease in family medicine: the importance of hypothesis generation. Med Decis Mak. 2009;29:282–90.
Zanchetta MB, Longobardi V, Bai JC. Bone and celiac disease. Curr Osteoporos Rep. 2016;14:43–8.
Corazza GR, Zoli G, Di Sabatino A, et al. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol. 1999;94:391–7.
Caraceni P, Benazzi B, Caio G, et al. Hyposplenism as a cause of pneumococcal meningoencephalitis in an adult patient with celiac disease. Ital J Med. 2011;5:124–7.
Di Sabatino A, Rosado MM, Cazzola P, et al. Splenic hypofunction and the spectrum of autoimmune and malignant complications in celiac disease. Clin Gastroenterol Hepatol. 2006;4:179–86.
Caio G, Riegler G, Patturelli M, et al. Pathophysiology of non-celiac gluten sensitivity: where are we now? Minerva Gastroenterol Dietol. 2017;63:16–21.
Villalta D, Tonutti E, Prause C, et al. IgG antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem. 2010;56:464–8.
Volta U, Molinaro N, De Franceschi L, et al. IgA anti-endomysial antibodies on human umbilical cord tissue for celiac disease screening: save both money and monkeys. Dig Dis Sci. 1995;40:1902–5.
Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000;31:513–9.
Salardi S, Volta U, Zucchini S, et al. Prevalence of celiac disease in children with type 1 diabetes mellitus increased in the mid-1990s: an 18-year longitudinal study based on anti-endomysial antibodies. J Pediatr Gastroenterol Nutr. 2008;46:612–4.
Zucchini L, Giusti D, Gatouillat G, et al. Interpretation of serological tests in the diagnosis of celiac disease: anti-deamidated gliadin peptide antibodies revisited. Autoimmunity. 2016;49:414–20.
Amarri S, Alvisi P, De Giorgio R, et al. Antibodies to deamidated gliadin peptides: an accurate predictor of coeliac disease in infancy. J Clin Immunol. 2013;33:1027–30.
Dipper CR, Maitra S, Thomas R, et al. Anti-tissue transglutaminase antibodies in the follow-up of adult coeliac disease. Aliment Parmacol Ther. 2009;30:236–44.
Choung RS, Khaleghi Rostamkolaei S, Ju JM, et al. Synthetic neoepitopes of the transglutaminase-deamidated gliadin complex as biomarkers for diagnosing and monitoring celiac disease. Gastroenterology. 2019;156:582–91.
Leonard MM, Weir DC, DeGroote M, et al. Value of IgA tTG in predicting mucosal recovery in children with celiac disease on a gluten-free diet. J Pediatr Gastroenterol Nutr. 2017;64:286–91.
Leonard MM, Fasano A. Zero, one, or two endoscopies to diagnose and monitor pediatric celiac disease? The jury is still out. J Pediatr Gastroenterol Nutr. 2017;65:270–1.
Oberhüber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94.
Corazza GR, Villanacci V. Coeliac disease: some considerations on the histological diagnosis. J Clin Pathol. 2005;58:573–4.
Oxentenko AS, Murray JA. Celiac disease: ten things that every gastroenterologist should know. Clin Gastroenterol Hepatol. 2015;13:1396–404.
Villanacci V, Ceppa P, Tavani E, et al. Coeliac disease: the histology report. Dig Liver Dis. 2011;43:385–95.
Rostami-Nejad M, Villanacci V, Hogg-Kollars S, et al. Endoscopic and histological pitfalls in the diagnosis of celiac disease: a multicentre study assessing the current practice. Rev Esp Enferm Dig. 2013;105:326–33.
Brown I, Mino-Kenudson M, Deshpande V, Lauwers GY. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa. Arch Pathol Lab Med. 2006;130:1020–5.
Biagi F, Bianchi PI, Campanella J, et al. The prevalence and the causes of minimal intestinal lesions in patients complaining of symptoms suggestive of enteropathy. A follow-up study. J Clin Pathol. 2008;61:1116–8.
Kakar S, Nehra V, Murray JA, et al. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosa architecture. Am J Gastroenterol. 2003;98:2027–33.
Upton MP. “Give us this day our daily bread”. Evolving concepts in celiac sprue. Arch Pathol Lab Med. 2008;132:1594–9.
Fernández-Bañares F, Carrasco A, García-Puig R, et al. Intestinal intraepithelial lymphocyte cytometric pattern is more accurate than subepithelial deposits of anti-tissue transglutaminase IgA for the diagnosis of celiac disease in lymphocytic enteritis. PLoS One. 2014;9:e101249.
Rostami K, Marsh MN, Johnson MW, et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080–6.
Marsh MN, Rostami K. What is a normal intestinal mucosa? Gastroenterology. 2016;151:784–8.
Marsh MN, Johnson WM, Rostami K. Mucosal histopathology in celiac disease: a rebuttal of Oberhuber's sub-division of Marsh III. Gastroenterol Hepatol Bed Bench. 2015;8:99–109.
Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76.
Volta U, Villanacci V. Celiac disease: diagnostic criteria in progress. Cell Mol Immunol. 2011;8:96–102.
Volta U, Caio G, Giancola F, et al. Features and progression of potential celiac disease in adults. Clin Gastroenterol Hepatol. 2016;14:686–93.
Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23.
Tosco A, Salvati VM, Auricchio R, et al. Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol. 2011;9:320–5.
Lionetti E, Castellaneta S, Pulvirenti A, et al. Prevalence and natural history of potential celiac disease in at-family-risk infants prospectively investigated from birth. J Pediatr. 2012;161:908–14.
Zanini B, Caselani F, Magni A, et al. Celiac disease with mild enteropathy is not mild disease. Clin Gastroenterol Hepatol. 2013;11:253–8.
Biagi F, Trotta L, Alfano C, et al. Prevalence and natural history of potential celiac disease in adult patients. Scand J Gastroenterol. 2013;48:537–42.
Auricchio R, Tosco A, Piccolo E, et al. Potential celiac children: 9-year follow-up on a gluten-containing diet. Am J Gastroenterol. 2014;109:913–21.
Kurppa K, Collin P, Lindfors K, et al. Spontaneous negative seroconversion of endomysial antibodies does not exclude subsequent celiac disease. J Pediatr Gastroenterol Nutr. 2011;53:576–9.
Rostami K, Kerckhaert J, Tiemessen R, et al. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888–94.
Shah VH, Rotterdam H, Kotler DP, et al. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717–20.
Greenson JK. The biopsy pathology of non-coeliac enteropathy. Histopathology. 2015;66:29–36.
De Gaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647–53.
Aziz I, Peerally MF, Barnes JH, et al. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000-2015). Gut. 2017;66:1563–72.
Dewar DH, Donnelly SC, McLaughlin SD, et al. Celiac disease: management of persistent symptoms in patients on a gluten-free diet. World J Gastroenterol. 2012;18:1348–56.
Rubio-Tapia A, Ludvigsson JF, Choung RS, et al. Increased mortality among men aged 50 years old or above with elevated IgA anti-transglutaminase antibodies: NHANES III. BMC Gastroenterol. 2016;16:136.
Biagi F, Gobbi P, Marchese A, et al. Low incidence but poor prognosis of complicated coeliac disease: a retrospective multicentre study. Dig Liver Dis. 2014;46:227–30.
Al-Toma A, Goerres MS, Meijer JW, et al. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol. 2006;4:315–9.
Di Sabatino A, Brunetti L, Carnevale Maffè G, et al. Is it worth investigating splenic function in patients with celiac disease? World J Gastroenterol. 2013;19:2313–8.
Roshan B, Leffler DA, Jamma S, et al. The incidence and clinical spectrum of refractory celiac disease in a north American referral center. Am J Gastroenterol. 2011;106:923–8.
Malamut G, Afchain P, Verkarre V, et al. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90.
Nasr I, Nasr I, Campling H, Ciclitira PJ. Approach to patients with refractory coeliac disease. F1000Res. 2016;5. https://doi.org/10.12688/f1000research.9051.1 .
Nasr I, Nasr I, Beyers C, et al. Recognising and managing refractory coeliac disease: a tertiary Centre experience. Nutrients. 2015;7:9896–907.
Williams MJ, Sutherland DH, Clark CG. Lymphosarcoma of the small intestine with a malabsorption syndrome and pneumatosis intestinalis. Report of a case with peroral jejunal biopsy. Gastroenterology. 1963;45:550e7.
Silano M, Volta U, Mecchia AM, et al. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol. 2007;7:8.
Malamut G, Cellier C. Complications of coeliac disease. Best Pract Res Clin Gastroenterol. 2015;29:451–8.
Cheminant M, Bruneau J, Malamut G, et al. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: a CELAC study. Gut. 2018. https://doi.org/10.1136/gutjnl-2018-317371 .
Ilus T, Kaukinen K, Virta LJ, et al. Incidence of malignancies in diagnosed celiac patients: a population-based estimate. Am J Gastroenterol. 2014;109:1471–7.
Caio G, Volta U, Ursini F, et al. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol. 2019;19:45.
Catassi C, Copparoni R, Corazza GR, et al. Protocollo per la diagnosi ed il follow-up della malattia celiaca. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale n.191 del 19/08/2015, pp. 148–58.
Tortora R, Capone P, De Stefano G, et al. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2015;41:352–9.
Reilly NR, Lebwohl B, Hultcrantz R, et al. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015;62:1405–11.
Laurikka P, Salmi T, Collin P, et al. Gastrointestinal symptoms in celiac disease patients on a long-term gluten-free diet. Nutrients. 2016;14:8.
Thompson T. Folate, iron, and dietary fiber contents of the gluten-free diet. J Am Diet Assoc. 2000;100:1389–96.
Carroccio A, Ambrosiano G, Di Prima L, et al. Clinical symptoms in celiac patients on a gluten-free diet. Scand J Gastroenterol. 2008;43:1315–21.
Valitutti F, Trovato CM, Montuori M, Cucchiara S. Pediatric celiac disease: follow-up in the spotlight. Adv Nutr. 2017;8:356–61.
Mubarak A, Oudshoorn JH, Kneepkens CM, et al. A child with refractory coeliac disease. J Pediatr Gastroenterol Nutr. 2011;53:216–8.
West J, Logan RF, Card TR, et al. Risk of vascular disease in adults with diagnosed coeliac disease: a population-based study. Aliment Pharmacol Ther. 2004;20:73–9.
Hallert C, Grant C, Grehn S, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–9.
Midhagen G, Hallert C. High rate of gastrointestinal symptoms in celiac patients living on a gluten-free diet: controlled study. Am J Gastroenterol. 2003;98:2023–6.
Roos S, Kärner A, Hallert C. Psychological well-being of adult coeliac patients treated for 10 years. Dig Liver Dis. 2006;38:177–80.
Aziz I, Evans KE, Papageorgiou V, Sanders DS. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31.
McCarville JL, Caminero A, Verdu EF. Pharmacological approaches in celiac disease. Curr Opin Pharmacol. 2015;25:7–12.
Leffler DA, Kelly CP, Green PH, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311–9.
Lähdeaho ML, Kaukinen K, Laurila K, et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology. 2014;146:1649–58.
Gottlieb K, Dawson J, Hussain F, Murray JA. Development of drugs for celiac disease: review of endpoints for phase 2 and 3 trials. Gastroenterol Rep. 2015;3:91–102.
Murray JA, Kelly CP, Green PHR, et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017;152:787–98.
Anderson RP, Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr Opin Immunol. 2013;25:410–7.
Lionetti E, Castellaneta S, Francavilla R, et al. Mode of delivery and risk of celiac disease: risk of celiac disease and age at gluten introduction cohort study. J Pediatr. 2017;184:81–6.
Koletzko S, Lee HS, Beyerlein A, et al. Cesarean section on the risk of celiac disease in the offspring: the teddy study. J Pediatr Gastroenterol Nutr. 2018;66:417–24.
Dydensborg Sander S, Hansen AV, Størdal K, et al. Mode of delivery is not associated with celiac disease. Clin Epidemiol. 2018;10:323–32.
Silvester JA, Leffler DA. Is autoimmunity infectious? The effect of gastrointestinal viral infections and vaccination on risk of celiac disease autoimmunity. Clin Gastroenterol Hepatol. 2017;15:703–5.
Leonard MM, Camhi S, Huedo-Medina TD, Fasano A. Celiac disease genomic, environmental, microbiome, and Metabolomic (CDGEMM) study design: approach to the future of personalized prevention of celiac disease. Nutrients. 2015;7:9325–36.
Hujoel IA, Van Dyke CT, Brantner T, et al. Natural history and clinical detection of undiagnosed coeliac disease in a north American community. Aliment Pharmacol Ther. 2018;47:1358–66.
Download references
This work was partially supported by the Fondo Incentivazione Ricerca (FIR) to R DeG, Fondi Ateneo per la Ricerca (FAR) to GC and RDe G (from University of Ferrara) and by the National Institute of Health grant R01DK104344 to AF.
Author information
Giacomo Caio and Umberto Volta these authors share co-first authorship.
Carlo Catassi and Alessio Fasano these authors share co-last authorship.
Authors and Affiliations
Department of Medical Sciences, University of Ferrara, Via Aldo Moro 8, Cona, 44124, Ferrara, Italy
Giacomo Caio & Roberto De Giorgio
Center for Celiac Research and Treatment, Massachusetts General Hospital, Boston, MA, 02114, USA
Giacomo Caio, Anna Sapone, Carlo Catassi & Alessio Fasano
Department of Medical and Surgical Sciences, University of Bologna, 40138, Bologna, Italy
Umberto Volta
Takeda Pharmaceuticals International Co, Cambridge, MA, 02139, USA
Anna Sapone & Daniel A. Leffler
Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, 02115, USA
Daniel A. Leffler
Department of Pediatrics, Center for Celiac Research, Università Politecnica delle Marche, 60121, Ancona, Italy
Carlo Catassi
You can also search for this author in PubMed Google Scholar
Contributions
Wrote the first draft of the manuscript: GC. Writing, correction and addition of fundamental insights to the manuscript: GC, UV, AS, DL, RDeG, CC, AF. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Giacomo Caio .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Caio, G., Volta, U., Sapone, A. et al. Celiac disease: a comprehensive current review. BMC Med 17 , 142 (2019). https://doi.org/10.1186/s12916-019-1380-z
Download citation
Received : 19 March 2019
Accepted : 27 June 2019
Published : 23 July 2019
DOI : https://doi.org/10.1186/s12916-019-1380-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Alternative treatment
- Clinical phenotypes
- Histopathological findings
- Pathogenesis
- Serological markers
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Gary Gray, who co-discovered possible celiac disease treatment, dies at 89
Gastroenterologist Gary Gray, part of Stanford Medicine for nearly 50 years, helped find the molecular cause of celiac disease and a potential treatment.
February 7, 2023 - By Emily Moskal

Gary Gray enjoyed photography, taking the photos for his family’s yearly holiday card. Courtesy of the Gray family
Gary Gray, MD, former chair of gastroenterology and hepatology at Stanford Medicine and an expert in celiac disease, died Oct. 31, 2022. He was 89.
Gray was the head of the Division of Gastroenterology and Hepatology for 17 years and the director of two previous organizations at Stanford Medicine: the Stanford Celiac Sprue Clinic and the Digestive Disease Center. He saw patients until around 2015.
“Dr. Gray was fully immersed in his work on the molecular chemistry of the gastrointestinal tract, becoming a major influence at Stanford Medicine and globally for many decades,” said Lloyd Minor , MD, dean of the Stanford School of Medicine . “We stand in the wake of his achievements.”
In 2002, Gray co-authored a paper on the molecular cause of celiac disease, an autoimmune condition that causes gluten intolerance. He studied a peptide — a short chain of amino acids, the building blocks of proteins — that causes the inflammatory response to gluten in affected patients. He and his co-authors found that an enzyme, prolyl endopeptidase, could be used as an oral therapy to detoxify gluten in celiac patients. Researchers plan to conduct late-stage clinical trials on the treatment in 2024.
“Gary was a scholar of the highest caliber,” said Chaitan Khosla , PhD, the Marc and Jennifer Lipschultz Director of the Stanford Innovative Medicines Accelerator and a professor of chemistry who was the senior author of the 2002 paper. “He was a true physician-scientist at a time when that wasn’t well recognized, let alone a thing to admire and strive for around the mid-’60s.”
Gray focused on the chemistry that allows food to be converted to energy in the small intestine, specializing in the enzymes sucrase, lactase and aminopeptidase, which have roles in digesting dietary sugars and proteins.
Early in his career, enzymes like these were hard to study because they are large proteins stuck inside cell membranes. But Gray pioneered a method to prepare these enzymes in a concentrated form directly from intestinal tissue, opening the door to studying their chemistry and regulation.
Gray published more than 140 peer-reviewed articles and textbook chapters on the topic, receiving more than 40 National Institutes of Health grants in roughly 12 years. He practiced translational medicine, which brought clinical applications to basic science research.
“I saw in Gary the ability to connect chemistry, biology, medicine and engineering in ways that were unique among his generation of physician-scientists,” Khosla said.
Decades-long career
Born June 4, 1933, in Seattle, Gray received his bachelor’s degrees in chemistry and mathematics from Seattle University in 1955, then completed his medical training at the University of Washington in 1959. He held two postdoctoral fellowships, one in biochemistry at the University of Chicago and another in gastroenterology at Boston University.
Gray became acquainted with tropical sprue , a rare digestive disorder that shares many features found in celiac disease, when he was working as the chief of metabolic studies for the U.S. Army Tropical Research Medical Laboratory in 1964 and 1965 in Puerto Rico. A year later, he arrived at Stanford Medicine.
“Gary’s contributions to basic science and gastroenterology were fundamental to building the School of Medicine to what it is today,” said Chris Cartwright , MD, emerita professor of gastroenterology. “He was a triple threat: an outstanding physician, scientist and mentor.”
He was a triple threat: an outstanding physician, scientist and mentor.
Gray was a chairman for the growth, development and nutrition section of the American Gastroenterological Association for two years ending in 1995; was a major contributor to the gastroenterology sections in Scientific American Medicine ; and was on the board of Gastroenterology and Viewpoints on Gastroenterology . He helped found the Celiac Sprue Research Foundation, a nonprofit that merged with another to form the Celiac Community Foundation of Northern California, and he was a visiting professor at many universities including Harvard and Yale.
He was inducted into the American Society for Clinical Investigation and the Association of American Physicians. He continued to publish 10 years after becoming an emeritus professorin 1999.
Eric Sibley , MD, PhD, a professor of pediatric gastroenterology who, as a postdoctoral scholar investigated lactase with Gray in 1993, said that Gray was also a champion of diversity, giving visibility and independence to Sibley and enabling him to become among the first Black professors on tenure track at Stanford Medicine.
“Gary was a strong advocate, mentor and sponsor,” Sibley said. “Even with him as my supervisor, it was hard not to be good friends with Gary. I would call him at different stages in my career, and he always was glad to take the time to talk.”
Besides research and mentorship, Gray also stood out with his devotion to his patients,according to Nielsen Fernandez-Becker , MD, PhD, a clinical associate professor of gastroenterology, who now treats many patients who were once Gray’s. Rather than relying on pathologists, Gray insisted on studying his own small intestinal samples to diagnose celiac disease. He was so devoted to his patients, Fernandez-Becker said, he gave them each his cell number.
Edifying passions
Outside of work, Gray had a love of hot rods. Khosla fondly remembers driving in Gray’s ’89 Corvette between the main Stanford University campus and the Stanford Research Park in Palo Alto.
“It was clear Gary enjoyed things not because of their external appearance but because of his ability to connect what they do to how they do it — and that was true with the human body and that was true with his Corvette,” Khosla said.
Gray also enjoyed photography, focusing on candid shots, and took the photos for his family’s yearly holiday card.
Gray had season tickets to the 49ers and the American Conservatory Theater, and he liked to spend time at his beach house in Pajaro Dunes, California, where he invited friends to an annual Thanksgiving celebration. Gathering at the dinner table is one of the fondest memories Michael Gray has of his dad.
“Having five boys at the dinner table was like herding cats, but my dad made it priority, and I think we’re better for it,” Michael Gray said.
David Gray, the eldest son, said that his family dinners were different from dinners at his friends’ homes. His father would quote The New York Times and hold engaging discussions about the latest news.
The dinners weren’t possible without the tight-knit connection between Gary Gray and his wife, Mary Gray. The two, high school sweethearts, were married for 62 years before shedied in 2021.
In addition to Michael and David, Gray is survived by sons Jeff, Peter and Jason as well as 12 grandchildren.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Gastrointestinal and hepatobiliary manifestations associated with untreated celiac disease in adults and children: a narrative overview.

1. Introduction
3. esophageal manifestations, 4. gastric manifestations, 5. intestinal manifestations, 6. pancreatic manifestations, 7. hepatic manifestations, 8. nutritional status, 9. pediatric development, 10. non-responsive celiac disease, 11. discussion, author contributions, data availability statement, conflicts of interest.
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022 , 399 , 2413–2426. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shiha, M.G.; Chetcuti Zammit, S.; Elli, L.; Sanders, D.S.; Sidhu, R. Updates in the diagnosis and management of coeliac disease. Best. Pract. Res. Clin. Gastroenterol. 2023 , 64–65 , 101843. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020 , 54 , 8–21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac Disease. Gastroenterol. Clin. N. Am. 2019 , 48 , 19–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jena, A.; Kumar-M, P.; Kumar, A.; Birda, C.L.; Choudhury, A.; Kumar, N.; Ramai, D.; Facciorusso, A.; Samanta, J. Liver abnormalities in celiac disease and response to gluten free diet: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2023 , 38 , 11–22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wieser, H.; Ciacci, C.; Gizzi, C.; Santonicola, A. Otorhinolaryngological Manifestations and Esophageal Disorders in Celiac Disease: A Narrative Review. J. Clin. Med. 2023 , 12 , 7036. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nachman, F.; Vázquez, H.; González, A.; Andrenacci, P.; Compagni, L.; Reyes, H.; Sugai, E.; Moreno, M.L.; Smecuol, E.; Hwang, H.J.; et al. Gastroesophageal Reflux Symptoms in Patients with Celiac Disease and the Effects of a Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2011 , 9 , 214–219. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mooney, P.D.; Evans, K.E.; Kurien, M.; Hopper, A.D.; Sanders, D.S. Gastro-oesophageal reflux symptoms and coeliac disease: No role for routine duodenal biopsy. Eur. J. Gastroenterol. Hepatol. 2015 , 27 , 692–697. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pellicano, R.; De Angelis, C.; Ribaldone, D.; Fagoonee, S.; Astegiano, M. 2013 Update on Celiac Disease and Eosinophilic Esophagitis. Nutrients 2013 , 5 , 3329–3336. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Visaggi, P.; Baiano Svizzero, F.; Savarino, E. Food elimination diets in eosinophilic esophagitis: Practical tips in current management and future directions. Best. Pract. Res. Clin. Gastroenterol. 2023 , 62–63 , 101825. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marsilio, I.; Maddalo, G.; Ghisa, M.; Savarino, E.V.; Farinati, F.; Zingone, F. The coeliac stomach: A review of the literature. Dig. Liver Dis. 2020 , 52 , 615–624. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ahonen, I.; Laurikka, P.; Koskimaa, S.; Huhtala, H.; Lindfors, K.; Kaukinen, K.; Kurppa, K.; Kivelä, L. Prevalence of vomiting and nausea and associated factors after chronic and acute gluten exposure in celiac disease. BMC Gastroenterol. 2023 , 23 , 301. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gabrieli, D.; Ciccone, F.; Capannolo, A.; Viscido, A.; Valerii, G.; Serva, D.; Necozione, S.; Coletti, G.; Calvisi, G.; Melideo, D.; et al. Subtypes of chronic gastritis in patients with celiac disease before and after gluten-free diet. United Eur. Gastroenterol. J. 2017 , 5 , 805–810. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tumgor, G.; Agin, M.; Doran, F.; Cetiner, S. Frequency of Celiac Disease in Children with Peptic Ulcers. Dig. Dis. Sci. 2018 , 63 , 2681–2686. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014 , 49 , 681–689. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vale, R.R.D.; Conci, N.d.S.; Santana, A.P.; Pereira, M.B.; Menezes, N.Y.H.; Takayasu, V.; Laborda, L.S.; da Silva, A.S.F. Celiac Crisis: An unusual presentation of gluten-sensitive enteropathy. Autopsy Case Rep. 2018 , 8 , e2018027. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Akman, S.; Sahaloglu, O.; Dalkan, C.; Bahceciler, N.N.; Arikan, C. Is celiac disease misdiagnosed in children with functional constipation? Turk. J. Gastroenterol. 2018 , 29 , 210–214. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Borkar, V.V.; Poddar, U.; Thakral, A.; Agarwal, J.; Srivastava, A.; Yachha, S.K.; Kumar, S. Intussusception in celiac disease: Is it a common feature in children? J. Gastroenterol. Hepatol. 2018 , 33 , 380–384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pelizzaro, F.; Marsilio, I.; Fassan, M.; Piazza, F.; Barberio, B.; D’odorico, A.; Savarino, E.V.; Farinati, F.; Zingone, F. The Risk of Malignancies in Celiac Disease—A Literature Review. Cancers 2021 , 13 , 5288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Volta, U.; Vincentini, O.; Quintarelli, F.; Felli, C.; Silano, M.; for the Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand. J. Gastroenterol. 2014 , 49 , 564–568. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kunovský, L.; Dítě, P.; Jabandžiev, P.; Eid, M.; Poredská, K.; Vaculová, J.; Sochorová, D.; Janeček, P.; Tesaříková, P.; Blaho, M.; et al. Causes of Exocrine Pancreatic Insufficiency Other Than Chronic Pancreatitis. J. Clin. Med. 2021 , 10 , 5779. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, C.; Barkin, J.A.; Barkin, J.S. Exocrine Pancreatic Insufficiency Is Common in Celiac Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2023 , 68 , 3421–3427. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alkhayyat, M.; Saleh, M.A.; Abureesh, M.; Khoudari, G.; Qapaja, T.; Mansoor, E.; Simons-Linares, C.R.; Vargo, J.; Stevens, T.; Rubio-Tapia, A.; et al. The Risk of Acute and Chronic Pancreatitis in Celiac Disease. Dig. Dis. Sci. 2021 , 66 , 2691–2699. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Narciso-Schiavon, J.L.; Schiavon, L.L. To screen or not to screen? Celiac antibodies in liver diseases. World J. Gastroenterol. 2017 , 23 , 776. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hoffmanová, I.; Sánchez, D.; Tučková, L.; Tlaskalová-Hogenová, H. Celiac Disease and Liver Disorders: From Putative Pathogenesis to Clinical Implications. Nutrients 2018 , 10 , 892. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marciano, F.; Savoia, M.; Vajro, P. Celiac disease-related hepatic injury: Insights into associated conditions and underlying pathomechanisms. Dig. Liver Dis. 2016 , 48 , 112–119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Castillo, N.E.; Vanga, R.R.; Theethira, T.G.; Rubio-Tapia, A.; Murray, J.A.; Villafuerte, J.; Bonder, A.; Mukherjee, R.; Hansen, J.; Dennis, M.; et al. Prevalence of Abnormal Liver Function Tests in Celiac Disease and the Effect of a Gluten-Free Diet in the US Population. Am. J. Gastroenterol. 2015 , 110 , 1216–1222. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benelli, E.; Naviglio, S.; De Leo, L.; Stera, G.; Giangreco, M.; Ronfani, L.; Villanacci, V.; Martelossi, S.; Ventura, A.; Not, T. Changing Epidemiology of Liver Involvement in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2019 , 68 , 547–551. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dutta, R.; Iqbal, A.; Das, P.; Palanichamy, J.K.; Singh, A.; Mehtab, W.; Chauhan, A.; Aggarwal, A.; Sreenivas, V.; Ahuja, V.; et al. Liver involvement in patients with coeliac disease: Proof of causality using IgA/anti-TG2 colocalisation techniques. J. Clin. Pathol. 2021 , 74 , 766–773. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rispo, A.; Imperatore, N.; Guarino, M.; Tortora, R.; Alisi, A.; Cossiga, V.; Testa, A.; Ricciolino, S.; Fiorentino, A.; Morisco, F. Metabolic-associated fatty liver disease (MAFLD) in coeliac disease. Liver Int. 2021 , 41 , 788–798. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Gut–liver axis: An immune link between celiac disease and primary biliary cirrhosis. Expert. Rev. Gastroenterol. Hepatol. 2013 , 7 , 253–261. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kamal, S.; Aldossari, K.K.; Ghoraba, D.; Abdelhakam, S.M.; Kamal, A.H.; Bedewi, M.; Nabegh, L.; Bahnasy, K.; Hafez, T. Clinicopathological and immunological characteristics and outcome of concomitant coeliac disease and non-alcoholic fatty liver disease in adults: A large prospective longitudinal study. BMJ Open Gastroenterol. 2018 , 5 , e000150. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Santonicola, A.; Wieser, H.; Gizzi, C.; Soldaini, C.; Ciacci, C. Associations between Celiac Disease, Extra-Gastrointestinal Manifestations, and Gluten-Free Diet: A Narrative Overview. Nutrients 2024 , 16 , 1814. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mędza, A.; Szlagatys-Sidorkiewicz, A. Nutritional Status and Metabolism in Celiac Disease: Narrative Review. J. Clin. Med. 2023 , 12 , 5107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bledsoe, A.C.; King, K.S.; Larson, J.J.; Snyder, M.; Absah, I.; Choung, R.S.; Murray, J.A. Micronutrient Deficiencies Are Common in Contemporary Celiac Disease Despite Lack of Overt Malabsorption Symptoms. Mayo Clin. Proc. 2019 , 94 , 1253–1260. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zingone, F.; Ciacci, C. The value and significance of 25(OH) and 1,25(OH) vitamin D serum levels in adult coeliac patients: A review of the literature. Dig. Liver Dis. 2018 , 50 , 757–760. [ Google Scholar ] [ CrossRef ]
- Lionetti, E.; Galeazzi, T.; Dominijanni, V.; Acquaviva, I.; Catassi, G.N.; Iasevoli, M.; Malamisura, B.; Catassi, C. Lower Level of Plasma 25-Hydroxyvitamin D in Children at Diagnosis of Celiac Disease Compared with Healthy Subjects: A Case-Control Study. J. Pediatr. 2021 , 228 , 132–137.e1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sulimani, R.A. Celiac disease and severe vitamin D deficiency: The case for anti-tissue transglutaminase antibody screening. Arch. Osteoporos. 2019 , 14 , 30. [ Google Scholar ] [ CrossRef ]
- Mahadev, S.; Laszkowska, M.; Sundström, J.; Björkholm, M.; Lebwohl, B.; Green, P.H.; Ludvigsson, J.F. Prevalence of Celiac Disease in Patients with Iron Deficiency Anemia—A Systematic Review with Meta-analysis. Gastroenterology 2018 , 155 , 374–382.e1. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Setavand, Z.; Ekramzadeh, M.; Honar, N. Evaluation of malnutrition status and clinical indications in children with celiac disease: A cross-sectional study. BMC Pediatr. 2021 , 21 , 147. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Giuseppe, R.; Bergomas, F.; Loperfido, F.; Giampieri, F.; Preatoni, G.; Calcaterra, V.; Cena, H. Could Celiac Disease and Overweight/Obesity Coexist in School-Aged Children and Adolescents? A Systematic Review. Child. Obes. 2024 , 20 , 48–67. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Auricchio, R.; Stellato, P.; Bruzzese, D.; Cielo, D.; Chiurazzi, A.; Galatola, M.; Castilljeo, G.; Escobar, P.C.; Gyimesi, J.; Hartman, C.; et al. Growth rate of coeliac children is compromised before the onset of the disease. Arch. Dis. Child 2020 , 105 , 964–968. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alamoudi, N.M.; Alsadat, F.A.; El-Housseiny, A.A.; Felemban, O.M.; Al Tuwirqi, A.A.; Mosli, R.H.; Saadah, O.I. Dental maturity in children with celiac disease: A case–control study. BMC Oral. Health 2020 , 20 , 311. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bayrak, N.A.; Volkan, B.; Haliloglu, B.; Kara, S.S.; Cayir, A. The effect of celiac disease and gluten-free diet on pubertal development: A two-center study. J. Pediatr. Endocrinol. Metab. 2020 , 33 , 409–415. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tye-Din, J.A. Evolution in Coeliac Disease Diagnosis and Management. JGH Open 2024 , 8 , e13107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Badran, Y.R.; Shih, A.; Leet, D.; Mooradian, M.J.; Coromilas, A.; Chen, J.; Kem, M.; Zheng, H.; Borowsky, J.; Misdraji, J.; et al. Immune checkpoint inhibitor-associated celiac disease. J. Immunother. Cancer 2020 , 8 , e000958. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alsaadi, D.; Shah, N.J.; Charabaty, A.; Atkins, M.B. A case of checkpoint inhibitor-induced celiac disease. J. Immunother. Cancer 2019 , 7 , 203. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Braun, D.S.; Patel, S.; Schwartz, A. Subclinical Celiac Disease Unmasked by Immune Checkpoint Inhibitor Therapy. J. Immunother. 2023 , 46 , 152–153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Penny, H.A.; Baggus, E.M.R.; Rej, A.; Snowden, J.A.; Sanders, D.S. Non-Responsive Coeliac Disease: A Comprehensive Review from the NHS England National Centre for Refractory Coeliac Disease. Nutrients 2020 , 12 , 216. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dicke, W.K. Investigation of the Harmful Effects of Certain Types of Cereals on Patients with Coeliac Disease. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 1950. [ Google Scholar ]
- Kaukinen, K.; Halme, L.; Collin, P.; Färkkilä, M.; Mäki, M.; Vehmanen, P.; Partanen, J.; Höckerstedt, K. Celiac disease in patients with severe liver disease: Gluten-free diet may reverse hepatic failure. Gastroenterology 2002 , 122 , 881–888. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Løvik, A.; Skodje, G.; Bratlie, J.; Brottveit, M.; Lundin, K.E.A. Diet Adherence and Gluten Exposure in Coeliac Disease and Self-Reported Non-Coeliac Gluten Sensitivity. Clin. Nutr. 2017 , 36 , 275–280. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-free Diet. J. Pediatr. Gastroenterol. Nutr. 2018 , 66 , 48–52. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Organ | Manifestations | Gluten-Free Diet Effect |
|---|---|---|
| Esophagus | GERD | Unclear |
| EoE | Potential benefit of food elimination diets (gluten/wheat) on clinical and histological remission. | |
| Stomach | Vomiting, nausea Indigestion, burping, belching, sensations of fullness | Symptom resolution |
| Gastritis and peptic ulcers | Unclear (gastritis and peptic ulcers) | |
| Intestine | Chronic diarrhea Constipation Abdominal pain Bloating and flatulence Intussusception (unclear) | Symptom resolution |
| Enteropathy-associated T-cell lymphoma, small bowel cancer | GFD may protect against or reduce the risk of malignancy (unclear) | |
| Pancreas | Exocrine pancreatic insufficiency (EPI). Acute pancreatitis and chronic pancreatitis | Untreated ced patients have an increased risk of EPI compared with those treated with a GFD |
| Liver | Hypertransaminasemia autoimmune hepatitis Primary biliary cirrhosis NAFLD | Normalized alt and resolution of the histological lesions (in many cases) |
| Nutritional Status | Malabsorption: macronutrients, micronutrients, fat-soluble vitamins, folic acid, vitamin B12, and minerals | GFD may prevent ongoing nutritional deficits |
| Pediatric Development | Growth impairment, failure to thrive Low body mass index, low body weight Overweight patients Delayed dental maturation Delayed sexual maturation. | Timely introduction of a strict GFD may ensure normal growth, dental, and sexual development. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Wieser, H.; Ciacci, C.; Soldaini, C.; Gizzi, C.; Santonicola, A. Gastrointestinal and Hepatobiliary Manifestations Associated with Untreated Celiac Disease in Adults and Children: A Narrative Overview. J. Clin. Med. 2024 , 13 , 4579. https://doi.org/10.3390/jcm13154579
Wieser H, Ciacci C, Soldaini C, Gizzi C, Santonicola A. Gastrointestinal and Hepatobiliary Manifestations Associated with Untreated Celiac Disease in Adults and Children: A Narrative Overview. Journal of Clinical Medicine . 2024; 13(15):4579. https://doi.org/10.3390/jcm13154579
Wieser, Herbert, Carolina Ciacci, Carlo Soldaini, Carolina Gizzi, and Antonella Santonicola. 2024. "Gastrointestinal and Hepatobiliary Manifestations Associated with Untreated Celiac Disease in Adults and Children: A Narrative Overview" Journal of Clinical Medicine 13, no. 15: 4579. https://doi.org/10.3390/jcm13154579
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

How Alzheimer’s disease could be reversed through lifestyle changes: new research
- A plant-based diet, strength training exercise and meditation can help reverse the disease’s symptoms, study says

It is not often you hear a hopeful story or read an encouraging study about dementia, given the sobering statistics about the disease.
Someone in the world develops dementia every three seconds, according to the non-profit Alzheimer’s Disease International. More than 55 million people worldwide lived with dementia in 2020, a number that is expected to double every 20 years, reaching 78 million in 2030 and 139 million in 2050.
Dr Dean Ornish, a professor of medicine at the University of California San Francisco and founder and president of the non-profit Preventive Medicine Research Institute, also in California, paints a brighter picture in a paper published in June in the journal Alzheimer’s Research & Therapy.

He suggests that radical lifestyle changes might not only slow the progression of dementia, but could even reverse it.
It sounds too good to be true given the less encouraging stories of a cure for the disease. But Ornish has reason to be optimistic.
“People talk about Alzheimer’s disease today just like they used to talk about heart disease,” he said in a recent podcast from the non-profit Us Against Alzheimer’s. “Forty-five years ago, the best that was hoped for was that the progression of heart disease might be slowed, not reversed altogether.”
It has since been proven to be reversible.

Ornish’s rules for health – and a healthy brain – as we age are simple: “Eat well, move more, stress less and love more.”
Ornish’s study – admittedly a small study sample – reviewed 51 adults in their 70s who all had signs of mild cognitive impairment or early Alzheimer’s.
At the end of the five-month study period, 70 per cent of the control group had worse cognitive function. Of the group who engaged in healthy interventions, 70 per cent were either stable or markedly improved.
The changes in some individuals were significant. Several participants whose symptoms had seen them stop reading began to read again; a musician remembered his music; a businessman who had not been able to manage his affairs was able to again; and a number of people who had struggled to follow complicated movie plots were able to enjoy films again.
The positive changes among participants who undertook interventions were astonishing given the short length of the study, the authors said.
Cici Zerbe, a Californian now in her mid-80s, was one of the patients in the control group. Her dementia appeared to worsen during the study but by the end, she committed to changing her lifestyle radically.
Five years later, in the documentary The Last Alzheimer’s Patient made for CNN by Dr Sanjay Gupta, Zerbe opens the door to Gupta and greets him by name.
In the documentary, Zerbe says her symptoms have been reversed after changes she made following the end of the study. She now walks every day and admits to a big change in her diet. “I haven’t had a breaded veal cutlet in years,” she laughs.
Gupta, who has Alzheimer’s in his family, is acutely aware of his own risk and had his profile analysed for the documentary.

In the course of assessing his risk, he spoke to neurologist and researcher Dr Richard Isaacson, director of the Florida Atlantic University Centre for Brain Health.
Isaacson, who also has the disease in his family, describes the recent case of Simon Nicholls. Nicholls lost his mother to Alzheimer’s and, at 55, was concerned about his memory. He spent a year under Isaacson’s care to manage his risk.
“His brain grew and his belly size got smaller,” Isaacson notes in the documentary.
Gupta’s tests were all normal. Isaacson calls him a “walking modifiable risk factor for Alzheimer’s” – in other words, he can keep managing his lifestyle to minimise his chance of developing the disease.
There were no signs or amyloid plaques or tau tangles in his brain, but as Gupta notes, he still has to watch for them.
Isaacson’s tips for best brain health in old age include eating a mostly plant-based diet; exercising regularly; taking brisk walks, particularly while wearing a weighted belt; and wearing a glucose monitor to keep an eye on blood sugar fluctuations.

Ornish – who designed a diet that experts recognised as being among the top healthy diets in the world in 2024 – says the problem with Alzheimer’s disease at the moment is that it can isolate a person, which further hastens the development of the disease.
Changing the way you live now – whatever your age – for better brain health later is a message of hope, he says, not despair.

A CDC Prevention Research Center
The Children’s Healthy Weight Research Group announces name change to the Community Health & Wellness Resource Team and launches new website
By iisabell

The Children’s Healthy Weight Research Group is excited to launch their new website and introduce their new team name and logo. After a thoughtful process, they are proud to call themselves the Community Health & Wellness Resource Team – a name that encompasses their mission, vision, and values to provide high-quality, evidence-based resources and research that support the health and well-being of all individuals.
Dr. Dianne S. Ward , the late founder and director of the Children’s Healthy Weight Research Group, played a key role in leading discussions surrounding this change. In this transition, they are guided by Dr. Ward’s vision for the future of the team and honoring her passion for ensuring optimal, equitable health and wellness.
The team’s early work and research focused on measuring and supporting children’s physical activity, nutrition, and health behaviors, particularly in care settings, with their hallmark tools including Go NAPSACC, the Environment and Policy Assessment and Observation (EPAO), and the Home Self-Administered Tool for Environmental Assessment of Activity and Diet (HomeSTEAD). With foundations in early childhood nutrition and physical activity, the Community Health & Wellness Resource Team’s research interests and work have expanded to support the overall health and wellness of caregivers and those in their care across the lifespan.
The Community Health & Wellness Resource Team will continue working toward their mission of supporting caregivers and those in their care by creating, evaluating, and implementing strategies to promote equitable health and wellness in early care and education settings and beyond. They look forward to their future work and impact and continuing Dr. Ward’s legacy of improving community health through evidence-based support, tools, and resources.
“The newly named Community Health & Wellness Resource Team has been a vital part of the Center for Health Promotion and Disease Prevention for over 20 years,” said Dr. Alice Ammerman, Director of the Center for Health Promotion and Disease Prevention. “The new name captures the very impressive growth and development of this group as well as their contributions to community health. Dr. Ward would be very proud of her team and their continued good work.”
This story is adapted from the original press release from CHWR.
Related Articles
- Share full article
Advertisement
Supported by
More Evidence Links Ultraprocessed Foods to Dementia
Recent research, including a new study on processed meat, has suggested these foods can affect brain health. Experts are trying to understand why.

By Dana G. Smith and Alice Callahan
People who regularly eat processed red meat, like hot dogs, bacon, sausage, salami and bologna, have a greater risk of developing dementia later in life. That was the conclusion of preliminary research presented this week at the Alzheimer’s Association International Conference.
The study tracked more than 130,000 adults in the United States for up to 43 years. During that period, 11,173 people developed dementia. Those who consumed about two servings of processed red meat per week had a 14 percent greater risk of developing dementia compared to those who ate fewer than three servings per month.
Eating unprocessed red meat, like steak or pork chops, did not significantly increase the risk for dementia, though people who ate it every day were more likely to report that they felt their cognition had declined than those who ate red meat less often. (The results of the study have not yet been published in a journal.)
The vast majority of processed meats are classified as “ ultraprocessed foods ” — products made with ingredients that you wouldn’t find in a home kitchen, like soy protein isolate, high fructose corn syrup, modified starches, flavorings or color additives. Many of these foods also have high levels of sugar, fat or sodium, which have long been known to adversely affect health.
Ultraprocessed foods, which also include items like sodas, flavored yogurts, instant soups and most breakfast cereals, make up a huge part of the American diet. They account for about 58 percent of the calories consumed by both children and adults, on average. In the last decade, researchers have linked these foods to health conditions including heart disease, Type 2 diabetes, obesity and some types of cancer and gastrointestinal diseases.
Now scientists are examining the connection between these foods and brain health.
What does the research suggest?
Several studies published in the past few years have found an association between eating more ultraprocessed foods and cognitive decline. In one study of more than 10,000 middle-aged adults in Brazil , for example, people who consumed 20 percent or more of their daily calories from ultraprocessed foods experienced more rapid cognitive decline, particularly on tests of executive functioning, over the course of eight years.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
- Remember me Not recommended on shared computers
Forgot your password?
Or sign in with one of these services
- Traveling with Celiac Disease
Welcome to Celiac.com!
You have found your celiac tribe! Join us and ask questions in our forum , share your story, and connect with others.
- Celiac.com Sponsor (A1): Celiac.com Sponsor (A1-M):
Celiac Disease and McDonald’s – Are There Safe Options?
By Fofala Eixdeal 1 hour ago in Traveling with Celiac Disease
Recommended Posts
I have celiac disease and am very careful about avoiding gluten. I’m a frequent traveler and sometimes end up at McDonald’s restaurant for a quick burger meal. I’ve heard mixed opinions about whether their food is safe for people with celiac disease.
Can anyone share their experiences or knowledge about McDonald’s menu options for those with celiac disease? Are there any specific items that are known to be gluten-free, or any tips on how to avoid cross-contamination?
Link to comment
Share on other sites.

30 minutes ago, Fofala Eixdeal said: I have celiac disease and am very careful about avoiding gluten. I’m a frequent traveler and sometimes end up at McDonald’s restaurant for a quick burger meal. I’ve heard mixed opinions about whether their food is safe for people with celiac disease. Can anyone share their experiences or knowledge about McDonald’s menu options for those with celiac disease? Are there any specific items that are known to be gluten-free, or any tips on how to avoid cross-contamination?
McDonald’s is not safe. Most fast food places aren’t. Chic-fi-let or Culver’s have gluten-free buns and they try to accommodate those with Celiac disease.
Create an account or sign in to comment
You need to be a member in order to leave a comment
Create an account
Sign up for a new account in our community. It's easy!
Already have an account? Sign in here.
- Celiac.com Sponsor (A17): Celiac.com Sponsor (A17): Celiac.com Sponsors (A17-M):
Recent Activity
What hams can you eat..

Newbie: Asymptomatic Celiac
- Celiac.com Sponsor (A19):
Member Statistics
- Total Members 123,673
- Most Online (within 30 mins) 7,748 September 18, 2023

- Celiac.com Sponsor (A20):
Forum Statistics
- Total Topics 120.6k
- Total Posts 1m
- Celiac.com Sponsor (A22):
Who's Online (See full list)
- There are no registered users currently online
- Celiac.com Sponsor (A21):
Upcoming Events
- Aug 13 0 National Celiac Association: Virtual Support Group: Living Gluten Free Zoom Meeting Aug/24 August 13, 2024 11:00 PM
- Aug 15 0 NCA ROCK Virtual Meet-Up for High Schoolers (October 2024) August 15, 2024 11:30 PM
- Aug 20 0 NCA ROCK Virtual Meet-Up for High Schoolers (August 2024) August 20, 2024 11:30 PM
- Sep 10 0 National Celiac Association: Virtual Support Group: Living Gluten Free Zoom Meeting Sep/24 September 10, 2024 11:00 PM
- Sep 17 0 NCA ROCK Virtual Meet-Up for High Schoolers (September 2024) September 17, 2024 11:30 PM
By OfcVal · Posted 55 minutes ago
By OfcVal · Posted 58 minutes ago
By Fofala Eixdeal · Posted 1 hour ago
By Mary J · Posted 8 hours ago
By trents · Posted 8 hours ago
- Existing user? Sign In
Forum Categories
- Coping with CD
- Pre-Diagnosis, Testing & Symptoms
- Post Diagnosis, Recovery & Treatment
- Related Issues & Disorders
- Dermatitis Herpetiformis
- GF Foods, Products, Shopping & Medications
- GF Recipes & Cooking Tips
- GF Restaurants
- Traveling with CD
- Sports and Fitness
- Introduce Yourself / Share Stuff
- Parents, Friends and Loved Ones
- Meet Up Room
- Publications & Publicity
- Food Intolerance & Leaky Gut
- Super Sensitive People
- Forum Technical Help
- Register - Join Us
- Latest Articles
All Categories
- View All Categories
- Safe & Unsafe Foods
- Celiac Disease FAQ
- Celiac Disease Basics
- Product Reviews
- GF Foods & Beverages
- Latest Research
Related Disorders
- All Disorder Categories
- Ataxia, Neuropathy & Nerve-Brain
- Bacterial Overgrowth & Candida
- Cancer & Lymphoma
- Casein & Cows Milk Intolerance
- Cognitive Impairment
- Crohn's Disease
- Dyspepsia & Acid Reflux
- Fertility, Pregnancy & Miscarriage
- Fibromyalgia
- Growth Hormone Deficiency
- Heart Issues
- Infertility & Impotency
- Inflammatory Bowel Disease
- Intestinal Permeability
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Migraine Headaches
- Multiple Sclerosis
- Osteoporosis & Osteomalacia
- Refractory CD
- Schizophrenia & Mental Issues
- Skin Problems & Rashes
- Thyroid & Pancreatic
- Other Disorders
- Diagnosis & Treatment
Miscellaneous
- All Misc. Categories
- Additional Concerns
- Projects, Fundraising, Epidemiology
- Publicity, Church, Pregnancy & Beer
- Labeling Regulations
- Codex Wheat Starch
- Podcast Edition
- Tax Deductions
Journal of Gluten Sensitivity
- GF Grains & Flours
- Kids and CD
- Allergy vs. Intolerance
- Support Groups
- Doctor Listing
Popular Articles
- About Celiac Disease
- Blood Tests
- Forbidden List
- GF Diet 101
- Nutrient Deficiencies
- Safe Alcohols & Drinks
- Genetic Testing
- Got Glutened?
- Ongoing Symptoms
- All Recipes
- All Countries
- American & British
- Chinese & Asian
- Indian & Middle Eastern
- Mexican & Spanish
- South American
- Biscuits, Buns & Rolls
- Flour Mixes
- Snacks & Appetizers
- Soups & Sauces
- Journal of Gluten Sensitivity - All Issues
- Spring 2024 Issue
- Winter 2024 Issue
- Autumn 2023 Issue
- Summer 2023 Issue
- Spring 2023 Issue
- Winter 2023 Issue
- Autumn 2022 Issue
- Summer 2022 Issue
- Spring 2022 Issue
- Winter 2022 Issue
- Autumn 2021 Issue
- Summer 2021 Issue
- Spring 2021 Issue
- Winter 2021 Issue
- Autumn 2020 Issue
- Summer 2020 Issue
- Spring 2020 Issue
- Winter 2020 Issue
- Autumn 2019 Issue
- Summer 2019 Issue
- Spring 2019 Issue
- Winter 2019 Issue
- Autumn 2018 Issue
- Summer 2018 Issue
- Spring 2018 Issue
- Winter 2018 Issue
- Autumn 2017 Issue
- Summer 2017 Issue
- Spring 2017 Issue
- Winter 2017 Issue
- Autumn 2016 Issue
- Summer 2016 Issue
- Spring 2016 Issue
- Winter 2016 Issue
- Autumn 2015 Issue
- Summer 2015 Issue
- Spring 2015 Issue
- Winter 2015 Issue
- Autumn 2014 Issue
- Summer 2014 Issue
- Spring 2014 Issue
- Winter 2014 Issue
- Autumn 2013 Issue
- Summer 2013 Issue
- Spring 2013 Issue
- Winter 2013 Issue
- Autumn 2012 Issue
- Summer 2012 Issue
- Spring 2012 Issue
- Winter 2012 Issue
- Autumn 2011 Issue
- Summer 2011 Issue
- Spring 2011 Issue
- Winter 2011 Issue
- Autumn 2010 Issue
- Summer 2010 Issue
- Spring 2010 Issue
- Winter 2010 Issue
- Autumn 2009 Issue
- Summer 2009 Issue
- Spring 2009 Issue
- Winter 2009 Issue
- Autumn 2008 Issue
- Summer 2008 Issue
- Spring 2008 Issue
- Winter 2008 Issue
- Autumn 2007 Issue
- Summer 2007 Issue
- Spring 2007 Issue
- Winter 2007 Issue
- Autumn 2006 Issue
- Summer 2006 Issue
- Spring 2006 Issue
- Winter 2006 Issue
- Autumn 2005 Issue
- Summer 2005 Issue
- Spring 2005 Issue
- Winter 2005 Issue
- Autumn 2004 Issue
- Summer 2004 Issue
- Spring 2004 Issue
- Winter 2004 Issue
- Autumn 2003 Issue
- Summer 2003 Issue
- Spring 2003 Issue
- Winter 2003 Issue
- Autumn 2002 Issue
- Summer 2002 Issue
- Join eNewsletter
- Advertising
- Terms of Use
- External Resources
- All Activity
- My Activity Streams
- Who's Online 30 Mins
- Leaderboard
- Create New...
- Election 2024
- Entertainment
- Newsletters
- Photography
- AP Buyline Personal Finance
- AP Buyline Shopping
- Press Releases
- Israel-Hamas War
- Russia-Ukraine War
- Global elections
- Asia Pacific
- Latin America
- Middle East
- Delegate Tracker
- AP & Elections
- 2024 Paris Olympic Games
- Auto Racing
- Movie reviews
- Book reviews
- Financial Markets
- Business Highlights
- Financial wellness
- Artificial Intelligence
- Social Media
Blood tests for Alzheimer’s may be coming to your doctor’s office. Here’s what to know
FILE - A doctor points to PET scan results that are part of a study on Alzheimer’s disease at Georgetown University Hospital, on Tuesday, May 19, 2015, in Washington. (AP Photo/Evan Vucci, File)
- Copy Link copied
WASHINGTON (AP) — New blood tests could help doctors diagnose Alzheimer’s disease faster and more accurately, researchers reported Sunday – but some appear to work far better than others.
It’s tricky to tell if memory problems are caused by Alzheimer’s. That requires confirming one of the disease’s hallmark signs — buildup of a sticky protein called beta-amyloid — with a hard-to-get brain scan or uncomfortable spinal tap. Many patients instead are diagnosed based on symptoms and cognitive exams.
Labs have begun offering a variety of tests that can detect certain signs of Alzheimer’s in blood. Scientists are excited by their potential but the tests aren’t widely used yet because there’s little data to guide doctors about which kind to order and when. The U.S. Food and Drug Administration hasn’t formally approved any of them and there’s little insurance coverage.
“What tests can we trust?” asked Dr. Suzanne Schindler, a neurologist at Washington University in St. Louis who’s part of a research project examining that. While some are very accurate, “other tests are not much better than a flip of a coin.”
Demand for earlier Alzheimer’s diagnosis is increasing
More than 6 million people in the United States and millions more around the world have Alzheimer’s, the most common form of dementia. Its telltale “biomarkers” are brain-clogging amyloid plaques and abnormal tau protein that leads to neuron-killing tangles.
This article is part of AP’s Be Well coverage, focusing on wellness, fitness, diet and mental health. Read more Be Well.
New drugs, Leqembi and Kisunla, can modestly slow worsening symptoms by removing gunky amyloid from the brain. But they only work in the earliest stages of Alzheimer’s and proving patients qualify in time can be difficult. Measuring amyloid in spinal fluid is invasive. A special PET scan to spot plaques is costly and getting an appointment can take months.
Even specialists can struggle to tell if Alzheimer’s or something else is to blame for a patient’s symptoms.
“I have patients not infrequently who I am convinced have Alzheimer’s disease and I do testing and it’s negative,” Schindler said.
New study suggests blood tests for Alzheimer’s can be simpler and faster
Blood tests so far have been used mostly in carefully controlled research settings. But a new study of about 1,200 patients in Sweden shows they also can work in the real-world bustle of doctors’ offices — especially primary care doctors who see far more people with memory problems than specialists but have fewer tools to evaluate them.
In the study, patients who visited either a primary care doctor or a specialist for memory complaints got an initial diagnosis using traditional exams, gave blood for testing and were sent for a confirmatory spinal tap or brain scan.
Blood testing was far more accurate, Lund University researchers reported Sunday at the Alzheimer’s Association International Conference in Philadelphia. The primary care doctors’ initial diagnosis was 61% accurate and the specialists’ 73% — but the blood test was 91% accurate, according to the findings, which also were published in the Journal of the American Medical Association.
Which blood tests for Alzheimer’s work best?
There’s almost “a wild West” in the variety being offered, said Dr. John Hsiao of the National Institute on Aging. They measure different biomarkers, in different ways.
Doctors and researchers should only use blood tests proven to have a greater than 90% accuracy rate, said Alzheimer’s Association chief science officer Maria Carrillo.
Today’s tests most likely to meet that benchmark measure what’s called p-tau217, Carrillo and Hsiao agreed. Schindler helped lead an unusual direct comparison of several kinds of blood tests, funded by the Foundation for the National Institutes of Health, that came to the same conclusion.
That type of test measures a form of tau that correlates with how much plaque buildup someone has, Schindler explained. A high level signals a strong likelihood the person has Alzheimer’s while a low level indicates that’s probably not the cause of memory loss.
Several companies are developing p-tau217 tests including ALZpath Inc., Roche, Eli Lilly and C2N Diagnostics, which supplied the version used in the Swedish study.
Who should use blood tests for Alzheimer’s?
Only doctors can order them from labs. The Alzheimer’s Association is working on guidelines and several companies plan to seek FDA approval, which would clarify proper use.
For now, Carrillo said doctors should use blood testing only in people with memory problems, after checking the accuracy of the type they order.
Especially for primary care physicians, “it really has great potential to help them in sorting out who to give a reassuring message and who to send on to memory specialists,” said Dr. Sebastian Palmqvist of Lund University, who led the Swedish study with Lund’s Dr. Oskar Hansson.
The tests aren’t yet for people who don’t have symptoms but worry about Alzheimer’s in the family — unless it’s part of enrollment in research studies, Schindler stressed.
That’s partly because amyloid buildup can begin two decades before the first sign of memory problems, and so far there are no preventive steps other than basic advice to eat healthy, exercise and get enough sleep. But there are studies underway testing possible therapies for people at high risk of Alzheimer’s, and some include blood testing.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
PRESS RELEASE
Research Advances at the Alzheimer’s Association International Conference
- GLP-1 drug may protect against dementia.
- Wildfire smoke and processed red meat raise risk.
- Blood tests could improve early diagnosis and speed access to treatment.
- Phase 2b clinical trial results of a GLP-1 agonist drug suggest it can protect against brain shrinkage associated with dementia.
- Breathing wildfire smoke and eating too much processed meat are bad for brain health and may raise the risk of cognitive decline and dementia.
- Some new Alzheimer’s blood tests are highly accurate and may improve recruiting for treatment trials and speed access to approved treatments.
AAIC is the premier annual conference for presentation and discussion of the latest Alzheimer’s and dementia research. This year’s conference in Philadelphia attracted nearly 14,000 registered attendees and included more than 5,260 scientific submissions.
GLP-1 Drug May Protect the Brain Liraglutide, a glucagon-like peptide drug (GLP-1), appears to protect the brain from shrinkage and slow cognitive decline , according to Phase 2b clinical trial results presented for the first time at AAIC 2024. GLP-1 agonists — like Ozempic and Zepbound — have been shown to help with diabetes and weight loss and reduce the risk of heart disease. The new research suggests they may also protect the brain.
The trial was led by researchers at the Imperial College, London, and included 204 patients living with Alzheimer’s who had a daily injection for one year: half received up to 1.8 mg of liraglutide and half received a placebo. The drug appeared to reduce shrinking in the parts of the brain that control memory, learning, language and decision-making by nearly 50% compared to placebo. People in the study who received liraglutide had slower decline in cognitive function after one year compared to those who received the placebo.
Wildfire Smoke Riskier for Brain than Other Types of Air Pollution Exposure to wildfire smoke may raise the risk of being diagnosed with dementia , according to a 10-year study of more than 1.2 million Californians, which was reported for the first time at AAIC 2024. Researchers determined the risk of dementia is notably stronger from wildfire smoke than from other sources of fine particulate matter air pollution (PM 2.5 ), such as motor vehicles and factories.
Researchers analyzed health records from more than 1.2 million socioeconomically diverse Kaiser Permanente Southern California members 60 or older. None of the members had been diagnosed with dementia at the beginning of the study. Researchers found an increased risk of dementia diagnosis due to wildfire PM 2.5 exposure. The risk of dementia was higher due to wildfire smoke, even with less exposure, than other sources of PM 2.5 . This is a serious problem, as air pollution produced by wildfires now accounts for more than 70% of total fine particulate matter exposure on poor air quality days in California.
Processed Red Meat May Raise Risk of Dementia; Swapping it for Nuts or Beans May Lower It People who eat about two servings a week of processed red meat have a 14% higher risk of dementia than those who eat less than about three servings a month, according to research reported for the first time at AAIC 2024. It also showed that replacing one serving of processed red meat every day with one serving of nuts and legumes can lower the risk of dementia by about 20%. Examples of processed red meat include bacon, hot dogs and sausage.
The research from the Harvard T.H. Chan School of Public Health, Boston, analyzed findings from more than 130,000 participants in the Nurses’ Health Study and Health Professionals Follow-Up Study tracked for up to 43 years. They based their findings on the participants’ answers to food-frequency questionnaires. Each additional daily serving of processed red meat was linked to an extra 1.6 years of cognitive aging for global cognition, and an extra 1.7 years of cognitive aging in verbal memory.
Blood Tests May Revolutionize Accuracy of Alzheimer’s Disease Diagnosis Blood tests for Alzheimer’s disease are moving closer to use in physicians’ offices. Research reported at AAIC 2024 suggests some of them (once confirmed) may make diagnoses much more accurate, support recruiting for future clinical trials, and help people get diagnosed and treated more quickly. Blood tests that assess phosphorylated tau (p-tau) protein to identify Alzheimer’s-related changes in the brain show the most promise.
One study from Lund University, Sweden, included 1,213 patients who were tested with the PrecivityAD2 test, and found it significantly outperformed clinicians who were not using a blood test. The test was about 90% accurate at identifying Alzheimer’s, while specialists at memory clinics were 73% accurate and primary care doctors were 63% accurate.
Findings of another study combining 2,718 cognitively unimpaired participants from 10 different studies suggest that levels of a marker called p-tau217 in plasma alone may be sufficient to select cognitively unimpaired amyloid-positive participants for clinical trials. A third study that used a forecasting model suggests that the use of high-performing blood tests in primary care could drastically reduce average wait times for Alzheimer’s diagnosis and treatment from nearly six years to less than six months.
About the Alzheimer's Association International Conference ® (AAIC ® ) The Alzheimer’s Association International Conference (AAIC) is the world’s largest gathering of researchers from around the world focused on Alzheimer’s and other dementias. As a part of the Alzheimer’s Association’s research program, AAIC serves as a catalyst for generating new knowledge about dementia and fostering a vital, collegial research community. Alzheimer’s Association: alz.org AAIC 2024: alz.org/aaic AAIC 2024 newsroom: alz.org/aaic/pressroom.asp AAIC 2024 hashtag: #AAIC24
About the Alzheimer's Association ® The Alzheimer’s Association is a worldwide voluntary health organization dedicated to Alzheimer’s care, support and research. Our mission is to lead the way to end Alzheimer’s and all other dementia — by accelerating global research, driving risk reduction and early detection, and maximizing quality care and support. Our vision is a world without Alzheimer’s and all other dementia®. Visit alz.org or call +1 800.272.3900.
Media Contacts: Alzheimer’s Association Media Line, +1 312.335.4078, [email protected] AAIC 2024 Press Office, [email protected]
Join the Conversation #AAIC25
AAIC Scientific Program Committee
The Student Experience
The ISTAART Experience
Conference Policies
Future Scientific Meetings
Abstract Submission Overview
Frequently Asked Questions
Submission Guidelines
Plenary Speakers
Scientific Sessions
Continuing Education
Sponsorship
Press Releases
News Highlights
Media Resources
Request for Press Credentials

We use cookies to improve your experience on this website. Learn about options for managing your personal data in our Privacy Policy .

IMAGES
VIDEO
COMMENTS
New research from Tampere University in Finland shows exciting progress in a new drug for celiac disease. This drug, ZED1227 could be used alongside a gluten-free diet to help manage celiac disease. In celiac disease, gluten causes inflammation and damage to the intestines.
Celiac disease, an autoimmune condition triggered by gluten consumption, has seen remarkable progress in recent research endeavors. This article delves into the latest breakthroughs, ongoing clinical trials, and the prospective landscape of celiac disease treatments. From innovative therapies to promising drug developments, the aim is to unravel the potential impact of these advancements on ...
New Hope for Celiac Disease: Promising Drug Shows Progress in Clinical Trials. New research from Tampere University in Finland shows exciting progress in a new drug for celiac disease. This drug, ZED1227 could be used alongside a gluten-free diet to help manage…. Continue Reading. 7/18/2024.
When blood tests suggest celiac disease, one third of patients don't get appropriate follow-up testing. Nearly one in three people who have positive blood tests for celiac disease don't go on to get the endoscopy and biopsy that confirms a celiac disease diagnosis, according to new research by Beyond Celiac. 04/25/2024.
Additionally, the study suggests that it is possible to measure intestinal tissue damage objectively and quantitatively in celiac disease research and clinical trials for new drugs. Tampere researchers plan to test whether molecular study is a sensitive tool for determining the benefits of ZED1227 in real life situations in which only small ...
Q: Has scientific research made any progress toward a cure or treatment for celiac disease? Until about 15 years ago, pharmaceutical companies showed little interest in drug development for celiac ...
Celiac Disease Clinical Trials. Researchers around the world are working to develop new treatments for celiac disease. As a person affected by celiac disease, you can play an important role in advancing research by participating in clinical trials. View our Clinical Trials Infographic to learn how clinical trials tie into to drug development process. ...
Wheat and other sources of gluten can spell trouble for people with celiac disease, and new research on the TG2 enzyme is helping scientists understand why. (Sleepy Claus/Flickr) Celiac disease affects around one in a hundred people worldwide , and those that have the autoimmune disorder have no choice but to stick to a gluten-free diet forever ...
Abstract. Coeliac disease is a common enteropathy that occurs in genetically susceptible individuals in response to the ingestion of gluten proteins present in wheat, rye and barley. Currently ...
Coeliac disease is an autoimmune disorder of the small intestine caused by immune cells reacting to ingested gluten present in wheat, rye and barley and also to small bowel tissue. It occurs in ...
A human in vitro model of coeliac disease comprising duodenal organoids that maintain both epithelium and an immune microenvironment finds a previously unsuspected role for IL-7 in gluten ...
The results of the new study, based on an analysis of the molecular activity of more than 10,000 genes, provide very strong evidence that the first successful drug to treat celiac disease may be ...
1. Introduction. Celiac disease (CD) is a chronic immune-mediated enteropathy triggered by exposure to dietary gluten in genetically predisposed individuals [1,2].The pooled global prevalence of CD has been reported to be approximately 1%, however, the prevalence values for CD varies in South America, Africa, North America, Asia, Europe, and Oceania; the prevalence is higher in female vs. male ...
Celiac disease: New findings on the effects of gluten. ScienceDaily . Retrieved August 2, 2024 from www.sciencedaily.com / releases / 2024 / 05 / 240516122605.htm
Results of a new phase 2 clinical trial using technology developed at Northwestern Medicine show it is possible to induce immune tolerance to gluten in individuals with celiac disease. The findings may pave the way for treated celiac patients to eventually tolerate gluten in their diet. After treatment with the technology, the patients were ...
Celiac disease (CD) is an autoimmune condition characterized by a specific serological and histological profile triggered by gluten ingestion in genetically predisposed individuals [].Gluten is the general term for alcohol-soluble proteins present in various cereals, including wheat, rye, barley, spelt, and kamut [].In recent years, there have been significant changes in the diagnosis ...
Celiac.com 04/01/2024 - Recent research has shed light on a concerning correlation between celiac disease and various reproductive disorders in women, emphasizing the importance of awareness and proactive healthcare measures for individuals living with this autoimmune condition. According to a study presented at the 2023 annual meeting of the ...
Celiac Disease causes malabsorption of vitamins and minerals. Most have low vitamin D. Ask your doctor to test her for vitamin D. A 2021 study found that 15.8% of 1,058 schoolchildren showed signs of iodine deficiency, with girls aged 10-12 being more likely to be deficient.
Gray became acquainted with tropical sprue, a rare digestive disorder that shares many features found in celiac disease, when he was working as the chief of metabolic studies for the U.S. Army Tropical Research Medical Laboratory in 1964 and 1965 in Puerto Rico. A year later, he arrived at Stanford Medicine.
Celiac disease (CeD) is a chronic inflammatory disease of the small intestine, produced by ingesting dietary gluten products in susceptible people. Gluten causes an impairment of the mucosal surface and, consequently, an abnormal absorption of nutrients. Although malabsorption of essential nutrients is a major risk factor for various CeD-associated morbidities, genetic, immunological, and ...
The Celiac Disease Foundation is now recruiting for the ACeD-it Study, a phase 1b/2 clinical trial evaluating the safety and tolerability of KAN-101 in people with celiac disease, and the SynCeD Study, a phase 2a clinical trial testing the ability of KAN-101 to protect the gut from gluten-induced damage. See if you qualify for the ACeD-it Study.
New Alzheimer's disease research finds that a plant-based diet, strength training exercise and meditation can help reverse the symptoms of the most common type of dementia.
Traveling with celiac disease is far from impossible, though it isn't without some challenges. You may have to research gluten-free restaurants before your trip or learn a few phrases in the local ...
Scientists have made another major stride toward the long-sought goal of diagnosing Alzheimer's disease with a simple blood test.On Sunday, a team of researchers reported that a blood test was ...
"The newly named Community Health & Wellness Resource Team has been a vital part of the Center for Health Promotion and Disease Prevention for over 20 years," said Dr. Alice Ammerman, Director of the Center for Health Promotion and Disease Prevention. "The new name captures the very impressive growth and development of this group as well ...
Research tracking more than 72,000 older adults from the United Kingdom for 10 years found that a diet containing 10 percent more ultraprocessed foods was associated with a 25 percent increased ...
The ILLUMINATE Studies are phase 2 clinical research studies evaluating an investigational medication designed to reduce or eliminate the negative immune response to gluten in adults with celiac disease. You may qualify for the ILLUMINATE Studies if you: If you are interested in participating, the study doctor or staff will review additional ...
I have celiac disease and am very careful about avoiding gluten. I'm a frequent traveler and sometimes end up at McDonald's restaurant for a quick burger meal. I've heard mixed opinions about whether their food is safe for people with celiac disease. Can anyone share their experiences or knowledg...
WASHINGTON (AP) — New blood tests could help doctors diagnose Alzheimer's disease faster and more accurately, researchers reported Sunday - but some appear to work far better than others.. It's tricky to tell if memory problems are caused by Alzheimer's. That requires confirming one of the disease's hallmark signs — buildup of a sticky protein called beta-amyloid — with a hard ...
New research results reported at AAIC 2024 are advancing what we know about risk, diagnosis and treatment of Alzheimer's disease and other dementias. ... — like Ozempic and Zepbound — have been shown to help with diabetes and weight loss and reduce the risk of heart disease. The new research suggests they may also protect the brain. The ...