Harvey Cushing/John Hay Whitney Medical Library
- Collections
- Research Help

YSN Doctoral Programs: Steps in Conducting a Literature Review
- Biomedical Databases
- Global (Public Health) Databases
- Soc. Sci., History, and Law Databases
- Grey Literature
- Trials Registers
- Data and Statistics
- Public Policy
- Google Tips
- Recommended Books
- Steps in Conducting a Literature Review
What is a literature review?
A literature review is an integrated analysis -- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question. That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
A literature review may be a stand alone work or the introduction to a larger research paper, depending on the assignment. Rely heavily on the guidelines your instructor has given you.
Why is it important?
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Discovers relationships between research studies/ideas.
- Identifies major themes, concepts, and researchers on a topic.
- Identifies critical gaps and points of disagreement.
- Discusses further research questions that logically come out of the previous studies.
APA7 Style resources
APA Style Blog - for those harder to find answers
1. Choose a topic. Define your research question.
Your literature review should be guided by your central research question. The literature represents background and research developments related to a specific research question, interpreted and analyzed by you in a synthesized way.
- Make sure your research question is not too broad or too narrow. Is it manageable?
- Begin writing down terms that are related to your question. These will be useful for searches later.
- If you have the opportunity, discuss your topic with your professor and your class mates.
2. Decide on the scope of your review
How many studies do you need to look at? How comprehensive should it be? How many years should it cover?
- This may depend on your assignment. How many sources does the assignment require?
3. Select the databases you will use to conduct your searches.
Make a list of the databases you will search.
Where to find databases:
- use the tabs on this guide
- Find other databases in the Nursing Information Resources web page
- More on the Medical Library web page
- ... and more on the Yale University Library web page
4. Conduct your searches to find the evidence. Keep track of your searches.
- Use the key words in your question, as well as synonyms for those words, as terms in your search. Use the database tutorials for help.
- Save the searches in the databases. This saves time when you want to redo, or modify, the searches. It is also helpful to use as a guide is the searches are not finding any useful results.
- Review the abstracts of research studies carefully. This will save you time.
- Use the bibliographies and references of research studies you find to locate others.
- Check with your professor, or a subject expert in the field, if you are missing any key works in the field.
- Ask your librarian for help at any time.
- Use a citation manager, such as EndNote as the repository for your citations. See the EndNote tutorials for help.
Review the literature
Some questions to help you analyze the research:
- What was the research question of the study you are reviewing? What were the authors trying to discover?
- Was the research funded by a source that could influence the findings?
- What were the research methodologies? Analyze its literature review, the samples and variables used, the results, and the conclusions.
- Does the research seem to be complete? Could it have been conducted more soundly? What further questions does it raise?
- If there are conflicting studies, why do you think that is?
- How are the authors viewed in the field? Has this study been cited? If so, how has it been analyzed?
Tips:
- Review the abstracts carefully.
- Keep careful notes so that you may track your thought processes during the research process.
- Create a matrix of the studies for easy analysis, and synthesis, across all of the studies.
- << Previous: Recommended Books
- Last Updated: Jun 20, 2024 9:08 AM
- URL: https://guides.library.yale.edu/YSNDoctoral

- University of Texas Libraries
Literature Reviews
Steps in the literature review process.
- What is a literature review?
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Analyze Results
- Librarian Support
- Artificial Intelligence (AI) Tools
- You may need to some exploratory searching of the literature to get a sense of scope, to determine whether you need to narrow or broaden your focus
- Identify databases that provide the most relevant sources, and identify relevant terms (controlled vocabularies) to add to your search strategy
- Finalize your research question
- Think about relevant dates, geographies (and languages), methods, and conflicting points of view
- Conduct searches in the published literature via the identified databases
- Check to see if this topic has been covered in other discipline's databases
- Examine the citations of on-point articles for keywords, authors, and previous research (via references) and cited reference searching.
- Save your search results in a citation management tool (such as Zotero, Mendeley or EndNote)
- De-duplicate your search results
- Make sure that you've found the seminal pieces -- they have been cited many times, and their work is considered foundational
- Check with your professor or a librarian to make sure your search has been comprehensive
- Evaluate the strengths and weaknesses of individual sources and evaluate for bias, methodologies, and thoroughness
- Group your results in to an organizational structure that will support why your research needs to be done, or that provides the answer to your research question
- Develop your conclusions
- Are there gaps in the literature?
- Where has significant research taken place, and who has done it?
- Is there consensus or debate on this topic?
- Which methodological approaches work best?
- For example: Background, Current Practices, Critics and Proponents, Where/How this study will fit in
- Organize your citations and focus on your research question and pertinent studies
- Compile your bibliography
Note: The first four steps are the best points at which to contact a librarian. Your librarian can help you determine the best databases to use for your topic, assess scope, and formulate a search strategy.
Videos Tutorials about Literature Reviews
This 4.5 minute video from Academic Education Materials has a Creative Commons License and a British narrator.
Recommended Reading
- Last Updated: Jul 30, 2024 9:33 AM
- URL: https://guides.lib.utexas.edu/literaturereviews

Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates
How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
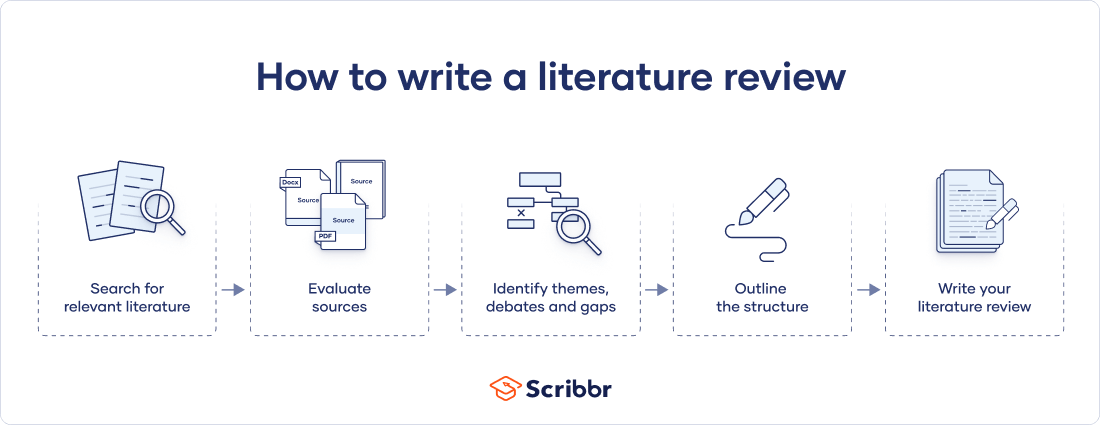
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Prevent plagiarism. Run a free check.
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved August 5, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Writing a Literature Review

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays). When we say “literature review” or refer to “the literature,” we are talking about the research ( scholarship ) in a given field. You will often see the terms “the research,” “the scholarship,” and “the literature” used mostly interchangeably.
Where, when, and why would I write a lit review?
There are a number of different situations where you might write a literature review, each with slightly different expectations; different disciplines, too, have field-specific expectations for what a literature review is and does. For instance, in the humanities, authors might include more overt argumentation and interpretation of source material in their literature reviews, whereas in the sciences, authors are more likely to report study designs and results in their literature reviews; these differences reflect these disciplines’ purposes and conventions in scholarship. You should always look at examples from your own discipline and talk to professors or mentors in your field to be sure you understand your discipline’s conventions, for literature reviews as well as for any other genre.
A literature review can be a part of a research paper or scholarly article, usually falling after the introduction and before the research methods sections. In these cases, the lit review just needs to cover scholarship that is important to the issue you are writing about; sometimes it will also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as assignments in a class or as publications. In a class, a lit review may be assigned to help students familiarize themselves with a topic and with scholarship in their field, get an idea of the other researchers working on the topic they’re interested in, find gaps in existing research in order to propose new projects, and/or develop a theoretical framework and methodology for later research. As a publication, a lit review usually is meant to help make other scholars’ lives easier by collecting and summarizing, synthesizing, and analyzing existing research on a topic. This can be especially helpful for students or scholars getting into a new research area, or for directing an entire community of scholars toward questions that have not yet been answered.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a place to discuss your goals, research methods, and conclusions separately from where you discuss the literature itself.
Introduction:
- An introductory paragraph that explains what your working topic and thesis is
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more often found in published, standalone literature reviews than in lit review sections in an article or research paper)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically Evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational patterns depending on what you are trying to accomplish with the review. Here are some examples:
- Chronological : The simplest approach is to trace the development of the topic over time, which helps familiarize the audience with the topic (for instance if you are introducing something that is not commonly known in your field). If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred (as mentioned previously, this may not be appropriate in your discipline — check with a teacher or mentor if you’re unsure).
- Thematic : If you have found some recurring central themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and the religious attitude towards women.
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
- Theoretical : In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theorical concepts to create a framework for your research.
What are some strategies or tips I can use while writing my lit review?
Any lit review is only as good as the research it discusses; make sure your sources are well-chosen and your research is thorough. Don’t be afraid to do more research if you discover a new thread as you’re writing. More info on the research process is available in our "Conducting Research" resources .
As you’re doing your research, create an annotated bibliography ( see our page on the this type of document ). Much of the information used in an annotated bibliography can be used also in a literature review, so you’ll be not only partially drafting your lit review as you research, but also developing your sense of the larger conversation going on among scholars, professionals, and any other stakeholders in your topic.
Usually you will need to synthesize research rather than just summarizing it. This means drawing connections between sources to create a picture of the scholarly conversation on a topic over time. Many student writers struggle to synthesize because they feel they don’t have anything to add to the scholars they are citing; here are some strategies to help you:
- It often helps to remember that the point of these kinds of syntheses is to show your readers how you understand your research, to help them read the rest of your paper.
- Writing teachers often say synthesis is like hosting a dinner party: imagine all your sources are together in a room, discussing your topic. What are they saying to each other?
- Look at the in-text citations in each paragraph. Are you citing just one source for each paragraph? This usually indicates summary only. When you have multiple sources cited in a paragraph, you are more likely to be synthesizing them (not always, but often
- Read more about synthesis here.
The most interesting literature reviews are often written as arguments (again, as mentioned at the beginning of the page, this is discipline-specific and doesn’t work for all situations). Often, the literature review is where you can establish your research as filling a particular gap or as relevant in a particular way. You have some chance to do this in your introduction in an article, but the literature review section gives a more extended opportunity to establish the conversation in the way you would like your readers to see it. You can choose the intellectual lineage you would like to be part of and whose definitions matter most to your thinking (mostly humanities-specific, but this goes for sciences as well). In addressing these points, you argue for your place in the conversation, which tends to make the lit review more compelling than a simple reporting of other sources.
- UWF Libraries
Literature Review: Conducting & Writing
- Steps for Conducting a Lit Review
1. Choose a topic. Define your research question.
2. decide on the scope of your review., 3. select the databases you will use to conduct your searches., 4. conduct your searches and find the literature. keep track of your searches, 5. review the literature..
- Finding "The Literature"
- Organizing/Writing
- APA Style This link opens in a new window
- Chicago: Notes Bibliography This link opens in a new window
- MLA Style This link opens in a new window
- Sample Literature Reviews
Disclaimer!!
Conducting a literature review is usually recursive, meaning that somewhere along the way, you'll find yourself repeating steps out-of-order.
That is actually a good sign.
Reviewing the research should lead to more research questions and those questions will likely lead you to either revise your initial research question or go back and find more literature related to a more specific aspect of your research question.
Your literature review should be guided by a central research question. Remember, it is not a collection of loosely related studies in a field but instead represents background and research developments related to a specific research question, interpreted and analyzed by you in a synthesized way.
- Make sure your research question is not too broad or too narrow. Is it manageable?
- Begin writing down terms that are related to your question. These will be useful for searches later.
- If you have the opportunity, discuss your topic with your professor.
How many studies do you need to look at? How comprehensive should it be? How many years should it cover?
Tip: This may depend on your assignment. How many sources does the assignment require?
Make a list of the databases you will search. Remember to include comprehensive databases such as WorldCat and Dissertations & Theses, if you need to.
Where to find databases:
- Find Databases by Subject UWF Databases categorized by discipline
- Find Databases via Research Guides Librarians create research guides for all of the disciplines on campus! Take advantage of their expertise and see what discipline-specific search strategies they recommend!
- Review the abstracts of research studies carefully. This will save you time.
- Write down the searches you conduct in each database so that you may duplicate them if you need to later (or avoid dead-end searches that you'd forgotten you'd already tried).
- Use the bibliographies and references of research studies you find to locate others.
- Ask your professor or a scholar in the field if you are missing any key works in the field.
- Use RefWorks to keep track of your research citations. See the RefWorks Tutorial if you need help.
Some questions to help you analyze the research:
- What was the research question of the study you are reviewing? What were the authors trying to discover?
- Was the research funded by a source that could influence the findings?
- What were the research methodologies? Analyze its literature review, the samples and variables used, the results, and the conclusions. Does the research seem to be complete? Could it have been conducted more soundly? What further questions does it raise?
- If there are conflicting studies, why do you think that is?
- How are the authors viewed in the field? Has this study been cited?; if so, how has it been analyzed?
Tips:
- Again, review the abstracts carefully.
- Keep careful notes so that you may track your thought processes during the research process.
- << Previous: Home
- Next: Finding "The Literature" >>
- Last Updated: Mar 22, 2024 9:37 AM
- URL: https://libguides.uwf.edu/litreview

- Become Involved |
- Give to the Library |
- Staff Directory |
- UNF Library
- Thomas G. Carpenter Library
Conducting a Literature Review
Steps in conducting a literature review.
- Benefits of Conducting a Literature Review
- Summary of the Process
- Additional Resources
- Literature Review Tutorial by American University Library
- The Literature Review: A Few Tips On Conducting It by University of Toronto
- Write a Literature Review by UC Santa Cruz University Library
Conducting a literature review involves using research databases to identify materials that cover or are related in some sense to the research topic. In some cases the research topic may be so original in its scope that no one has done anything exactly like it, so research that is at least similar or related will provide source material for the literature review. The selection of databases will be driven by the subject matter and the scope of the project.
Selecting Databases -- Most academic libraries now provide access to a majority of their databases and their catalog via a so-called discovery tool. A discovery tool makes searching library systems more "Google-like" in that even the simplest of queries can be entered and results retrieved. However, many times the results are also "Google-like" in the sheer quantity of items retrieved. While a discovery tool can be invaluable for quickly finding a multitude of resources on nearly any topic, there are a number of considerations a researcher should keep in mind when using a discovery tool, especially for the researcher who is attempting a comprehensive literature review.
No discovery tool works with every database subscribed to by a library. Some libraries might subscribe to two or three hundred different research databases covering a large number of subject areas. Competing discovery systems might negotiate agreements with different database vendors in order to provide access to a large range of materials. There will be other vendors with whom agreements are not forthcoming, therefore their materials are not included in the discovery tool results. While this might be of only minor concern for a researcher looking to do a fairly limited research project, the researcher looking to do a comprehensive review of the literature in preparation for writing a master's thesis or a doctoral dissertation will run the risk of missing some materials by limiting the search just to a particular library's discovery system. If only one system covered everything that a researcher could possibly need, libraries would have no need to subscribe to hundreds of different databases. The reality is that no one tool does it all. Not even Google Scholar.
Book collections might be excluded from results delivered by a discovery tool. While many libraries are making results from their own catalogs available via their discovery tools, they might not cover books that are discoverable from other library collections, thus making a search of book collections incomplete. Most libraries subscribe to an international database of library catalogs known as WorldCat. This database will provide comprehensive coverage of books, media, and other physical library materials available in libraries worldwide.
Features available in a particular database might not be available in a discovery tool. Keep in mind that a discovery tool is a search system that enables searching across content from numerous individual databases. An individual database might have search features that cannot be provided through a discovery tool, since the discovery tool is designed to accommodate a large number of systems with a single search. For example, the nursing database CINAHL includes the ability to limit a search to specific practice areas, to limit to evidence-based practice, to limit to gender, and to search using medical subject headings, among other things, all specialized facets that are not available in a discovery tool. To have these advanced capabilities, a researcher would need to go directly to CINAHL and search it natively.
Some discovery tools are set, by default, to limit search results to those items directly available through a particular library's collections. While many researchers will be most concerned with what is immediately available to them at their own library, a researcher concerned with finding everything that has been done on a particular topic will need to go beyond what's available at his or her home library and include materials that are available elsewhere. Master's and doctoral candidates should take care to notice if their library's discovery tool automatically limits to available materials and broaden the scope to include ALL materials, not just those available.
With the foregoing in mind, a researcher might start a search by using the library's discovery tool and then follow up by reviewing which databases have been included in the search and, more importantly, which databases have not been included. Most libraries will facilitate locating its individual databases through a subject arrangement of some kind. Once those databases that are not discoverable have been identified, the researcher would do well to search them individually to find out if other materials can be identified outside of the discovery tool. One additional tool that a doctoral researcher should of necessity include in a search is ISI's Web of Knowledge . The two major systems searchable within ISI's Web are the Social Sciences Citation Index and the Science Citation Index . The purpose of these two systems is to enable a researcher to determine what research has been cited over the years by any number of researchers and how many times it has been cited.
Formulating an Effective Search Strategy -- Key to performing an effective literature review is selecting search terms that will effectively identify materials that are relevant to the research topic. An initial strategy for selecting search terminology might be to list all possible relevant terms and their synonyms in order to have a working vocabulary for use in the research databases. While an individual subject database will likely use a "controlled vocabulary" to index articles and other materials that are included in the database, the same vocabulary might not be as effective in a database that focuses on a different subject area. For example, terminology that is used frequently in psychological literature might not be as effective in searching a human resources management database. Brainstorming the topic before launching into a search will help a researcher arrive at a good working vocabulary to use when probing the databases for relevant literature.
As materials are identified with the initial search, the researcher will want to keep track of other terminology that could be of use in performing additional searches. Sometimes the most effective search terminology can be found by reading the abstracts of relevant materials located through a library's research databases. For example, an initial search on the concept of "mainstreaming" might lead the researcher to articles that discuss mainstreaming but which also look into the concept of "inclusion" in education. While the terms mainstreaming and inclusion are sometimes used synonymously, they really embody two different approaches to working with students having special needs. Abstracts of articles located in the initial search on mainstreaming will uncover related concepts such as inclusion and help a researcher develop a better, more effective vocabulary for fleshing out the literature review.
In addition to searching using key concepts aligned with the research topic, a researcher likely also will want to search for additional materials produced by key authors who are identified in the initial searches. As a researcher reviews items retrieved in the initial stages of the survey, he or she will begin to notice certain authors coming up over and over in relation to the topic. To make sure that no stone is left unturned, it would be advisable to search the available, relevant library databases for other materials by those key authors, just to make sure something of importance has not been missed. A review of the reference lists for each of the items identified in the search will also help to identify key literature that should be reviewed.
Locating the Materials and Composing the Review -- In many cases the items identified through the library's databases will also be available online through the same or related databases. This, however, is not always the case. When materials are not available online, the researcher should check the library's physical collections (print, media, etc.) to determine if the items are available in the library, itself. For those materials not physically available in the home library, the researcher will use interlibrary loan to procure copies from other libraries or services. While abstracts are extremely useful in identifying the right types of materials, they are no substitute for the actual items, themselves. The thorough researcher will make sure that all the key literature has been retrieved and read thoroughly before proceeding too far with the original research.
The end result of the literature review is a discussion of the central themes in the research and an overview of the significant studies located by the researcher. This discussion serves as the lead section of a paper or article that reports the findings of an original research study and sets the stage for presentation of the original study by providing a review of research that has been conducted prior to the current study. As the researcher conducts his or her own study, other relevant materials might enter into the professional literature. It is the researcher's responsibility to update the literature review with newly released information prior to completing his or her own study.
Updating the Initial Search -- Most research projects will take place over a period of time and are not completed in the short term. Especially in the case of master's and doctoral projects, the research process might take a year or several years to complete. During this time, it will be important for the researcher to periodically review the research that has been going on at the same time as his or her own research. Revisiting the search strategies employed in the initial pass of the ltierature will turn up any new studies that might have come to light since the initial search. Fortunately, most research databases and discovery systems provide researchers with the means for automatically notifying them when new materials matching the search strategy have entered the system. This requires that a researcher sign up for a personal "account" with the database in order to save his or her searches and set up "alerts" when new materials come online. Setting up an account does not involve charges to the researcher; this is all a part of the cost borne by the home library in providing access to the databases.
- << Previous: Benefits of Conducting a Literature Review
- Next: Summary of the Process >>
- Last Updated: Aug 29, 2022 8:54 AM
- URL: https://libguides.unf.edu/litreview
- UConn Library
- Literature Review: The What, Why and How-to Guide
- Introduction
Literature Review: The What, Why and How-to Guide — Introduction
- Getting Started
- How to Pick a Topic
- Strategies to Find Sources
- Evaluating Sources & Lit. Reviews
- Tips for Writing Literature Reviews
- Writing Literature Review: Useful Sites
- Citation Resources
- Other Academic Writings
What are Literature Reviews?
So, what is a literature review? "A literature review is an account of what has been published on a topic by accredited scholars and researchers. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries." Taylor, D. The literature review: A few tips on conducting it . University of Toronto Health Sciences Writing Centre.
Goals of Literature Reviews
What are the goals of creating a Literature Review? A literature could be written to accomplish different aims:
- To develop a theory or evaluate an existing theory
- To summarize the historical or existing state of a research topic
- Identify a problem in a field of research
Baumeister, R. F., & Leary, M. R. (1997). Writing narrative literature reviews . Review of General Psychology , 1 (3), 311-320.
What kinds of sources require a Literature Review?
- A research paper assigned in a course
- A thesis or dissertation
- A grant proposal
- An article intended for publication in a journal
All these instances require you to collect what has been written about your research topic so that you can demonstrate how your own research sheds new light on the topic.
Types of Literature Reviews
What kinds of literature reviews are written?
Narrative review: The purpose of this type of review is to describe the current state of the research on a specific topic/research and to offer a critical analysis of the literature reviewed. Studies are grouped by research/theoretical categories, and themes and trends, strengths and weakness, and gaps are identified. The review ends with a conclusion section which summarizes the findings regarding the state of the research of the specific study, the gaps identify and if applicable, explains how the author's research will address gaps identify in the review and expand the knowledge on the topic reviewed.
- Example : Predictors and Outcomes of U.S. Quality Maternity Leave: A Review and Conceptual Framework: 10.1177/08948453211037398
Systematic review : "The authors of a systematic review use a specific procedure to search the research literature, select the studies to include in their review, and critically evaluate the studies they find." (p. 139). Nelson, L. K. (2013). Research in Communication Sciences and Disorders . Plural Publishing.
- Example : The effect of leave policies on increasing fertility: a systematic review: 10.1057/s41599-022-01270-w
Meta-analysis : "Meta-analysis is a method of reviewing research findings in a quantitative fashion by transforming the data from individual studies into what is called an effect size and then pooling and analyzing this information. The basic goal in meta-analysis is to explain why different outcomes have occurred in different studies." (p. 197). Roberts, M. C., & Ilardi, S. S. (2003). Handbook of Research Methods in Clinical Psychology . Blackwell Publishing.
- Example : Employment Instability and Fertility in Europe: A Meta-Analysis: 10.1215/00703370-9164737
Meta-synthesis : "Qualitative meta-synthesis is a type of qualitative study that uses as data the findings from other qualitative studies linked by the same or related topic." (p.312). Zimmer, L. (2006). Qualitative meta-synthesis: A question of dialoguing with texts . Journal of Advanced Nursing , 53 (3), 311-318.
- Example : Women’s perspectives on career successes and barriers: A qualitative meta-synthesis: 10.1177/05390184221113735
Literature Reviews in the Health Sciences
- UConn Health subject guide on systematic reviews Explanation of the different review types used in health sciences literature as well as tools to help you find the right review type
- << Previous: Getting Started
- Next: How to Pick a Topic >>
- Last Updated: Sep 21, 2022 2:16 PM
- URL: https://guides.lib.uconn.edu/literaturereview

Literature Review
- Steps for Conducting a Lit Review
- Finding "The Literature"
- Organizing/Writing
- Sample Literature Reviews
- FAMU Writing Center
1. Choose a topic. Define your research question.
Your literature review should be guided by a central research question. Remember, it is not a collection of loosely related studies in a field but instead represents background and research developments related to a specific research question, interpreted and analyzed by you in a synthesized way.
- Make sure your research question is not too broad or too narrow. Is it manageable?
- Begin writing down terms that are related to your question. These will be useful for searches later.
- If you have the opportunity, discuss your topic with your professor.
2. Decide on the scope of your review.
How many studies do you need to look at? How comprehensive should it be? How many years should it cover?
Tip: This may depend on your assignment. How many sources does the assignment require?
3. Select the databases you will use to conduct your searches.
Make a list of the databases you will search.
- Look at the Library's research guides in your discipline to select discipline-specific databases. Don't forget to look at books!
- Make an appointment with or contact your subject librarian to make sure you aren't missing major databases.
4. Conduct your searches and find the literature. Keep track of your searches!
Tips:
- Review the abstracts of research studies carefully. This will save you time.
- Write down the searches you conduct in each database so that you may duplicate them if you need to later (or avoid dead-end searches that you'd forgotten you'd already tried).
- Use the bibliographies and references of research studies you find to locate others.
- Ask your professor or a scholar in the field if you are missing any key works in the field.
- Use RefWorks to keep track of your research citations. See the RefWorks Tutorial if you need help.
5. Review the literature.
Some questions to help you analyze the research:
- What was the research question of the study you are reviewing? What were the authors trying to discover?
- Was the research funded by a source that could influence the findings?
- What were the research methodologies? Analyze its literature review, the samples and variables used, the results, and the conclusions. Does the research seem to be complete? Could it have been conducted more soundly? What further questions does it raise?
- If there are conflicting studies, why do you think that is?
- How are the authors viewed in the field? Has this study been cited?; if so, how has it been analyzed?
- Again, review the abstracts carefully.
- Keep careful notes so that you may track your thought processes during the research process.
Composing your literature review
O nce you've settled on a general pattern of organization, you're ready to write each section. There are a few guidelines you should follow during the writing stage. Here is a sample paragraph from a literature review about sexism and language to illuminate the following discussion:
However, other studies have shown that even gender-neutral antecedents are more likely to produce masculine images than feminine ones (Gastil, 1990). Hamilton (1988) asked students to complete sentences that required them to fill in pronouns that agreed with gender-neutral antecedents such as "writer," "pedestrian," and "persons." The students were asked to describe any image they had when writing the sentence. Hamilton found that people imagined 3.3 men to each woman in the masculine "generic" condition and 1.5 men per woman in the unbiased condition. Thus, while ambient sexism accounted for some of the masculine bias, sexist language amplified the effect. (Source: Erika Falk and Jordan Mills, "Why Sexist Language Affects Persuasion: The Role of Homophily, Intended Audience, and Offense," Women and Language19:2.
Use evidence
In the example above, the writers refer to several other sources when making their point. A literature review in this sense is just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence to show that what you are saying is valid.
Be selective
Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the review's focus, whether it is thematic, methodological, or chronological.
Use quotes sparingly
Falk and Mills do not use any direct quotes. That is because the survey nature of the literature review does not allow for in-depth discussion or detailed quotes from the text. Some short quotes here and there are okay, though if you want to emphasize a point, or if what the author said just cannot be rewritten in your own words. Notice that Falk and Mills do quote certain terms that were coined by the author, not common knowledge, or taken directly from the study. But if you find yourself wanting to put in more quotes, check with your instructor.
Summarize and synthesize
Remember to summarize and synthesize your sources within each paragraph as well as throughout the review. The authors here recapitulate important features of Hamilton's study, but then synthesize it by rephrasing the study's significance and relating it to their own work.
Keep your own voice
While the literature review presents others' ideas, your voice (the writer's) should remain front and center. Notice that Falk and Mills weave references to other sources into their own text, but they still maintain their own voice by starting and ending the paragraph with their own ideas and their own words. The sources support what Falk and Mills are saying.
Use caution when paraphrasing
When paraphrasing a source that is not your own, be sure to represent the author's information or opinions accurately and in your own words. In the preceding example, Falk and Mills either directly refer in the text to the author of their source, such as Hamilton, or they provide ample notation in the text when the ideas they are mentioning are not their own, for example, Gastil's. For more information, please see our handout on plagiarism .
- << Previous: Home
- Next: Finding "The Literature" >>
- Last Updated: Oct 20, 2022 11:24 AM
- URL: https://library.famu.edu/literaturereview
- Research Guides
- University Libraries
- Advanced Research Topics
Common Paper Types
- Literature Review
- Scoping Review
- Systematic Review
- Author Profile
Understanding Literature Reviews
I. Getting Started with a Workshop Video (Highly recommended!)
- Searching for Literature Reviews: Before You Write, You Have to Find https://www.youtube.com/watch?v=9la5ytz9MmM
A lecture by the Writing Center, TAMU.
II. What is a Literature Review?
- Generally, the purpose of a review is to analyze critically a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles. <http://writing.wisc.edu/Handbook/ReviewofLiterature.html>
- A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information. It might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. And depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant. < http://writingcenter.unc.edu/resources/handouts-demos/specific-writing-assignments/literature-reviews >
- A literature review is a body of text that aims to review the critical points of current knowledge including substantive findings as well as theoretical and methodological contributions to a particular topic...Its ultimate goal is to bring the reader up to date with current literature on a topic and forms the basis for another goal, such as future research that may be needed in the area. <http://en.wikipedia.org/wiki/Literature_review>
III. What Major Steps Literature Reviews Require?
- 1. Develop a review protocol. Protocols define the scope of studies that will be reviewed, the process through which studies will be identified, and the outcomes that will be examined. Protocols also specify the time period during which relevant studies will have been conducted, the outcomes to be examined in the review, and keyword strategies for the literature search. 2. Identify relevant studies, often through a systematic search of the literature. 3. Screen studies for relevance and the adequacy of study design, implementation, and reporting. 4. Retrieve and summarize information on the intervention studied, the study characteristics, and the study findings. 5. Combine findings within studies and across studies when relevant. < http://ies.ed.gov/ncee/wwc/pdf/reference_resources/wwc_procedures_v2_1_standards_handbook.pdf>
- The basic stages in a typical research project are: i) identify your topic of interest, ii) perform a literature review, iii) generate related questions, iv) state your unsolved problem or hypothesis, v) find or develop a solution, and vi) document your results.
- The four stages required: Problem formulation—which topic or field is being examined and what are its component issues? Literature search—finding materials relevant to the subject being explored Data evaluation—determining which literature makes a significant contribution to the understanding of the topic Analysis and interpretation—discussing the findings and conclusions of pertinent literature < http://library.ucsc.edu/help/howto/write-a-literature-review#components >
IV. What Basic Elements Comprise a Literature Review?
- An overview of the subject, issue or theory under consideration, along with the objectives of the literature review
- Division of works under review into categories (e.g. those in support of a particular position, those against, and those offering alternative theses entirely)
- Explanation of how each work is similar to and how it varies from the others
- Conclusions as to which pieces are best considered in their argument, are most convincing of their opinions, and make the greatest contribution to the understanding and development of their area of research
< http://library.ucsc.edu/help/howto/write-a-literature-review#components > V. Which Citation Tool Are You Going to Use to Manage the Search Results?
- Choose your citation tool before conducing your literature reviews. If you decide to use RefWorks , the information can be found at http://tamu.libguides.com/refworks .
VII. Other Useful Guides
- Literature Reviews (University of North Carolina at Chapel Hill)
- The Literature Review: A Few Tips On Conducting It
- How to Write a Literature Review (UCSC)
- Learn how to write a review of literature (WISC)
- Reviewing the Literature
- Next: Scoping Review >>
- Last Updated: Aug 5, 2024 7:43 AM
- URL: https://tamu.libguides.com/c.php?g=1415100
The Sheridan Libraries
- Write a Literature Review
- Sheridan Libraries
- Evaluate This link opens in a new window
What Will You Do Differently?
Please help your librarians by filling out this two-minute survey of today's class session..
Professor, this one's for you .
Introduction
Literature reviews take time. here is some general information to know before you start. .
- VIDEO -- This video is a great overview of the entire process. (2020; North Carolina State University Libraries) --The transcript is included --This is for everyone; ignore the mention of "graduate students" --9.5 minutes, and every second is important
- OVERVIEW -- Read this page from Purdue's OWL. It's not long, and gives some tips to fill in what you just learned from the video.
- NOT A RESEARCH ARTICLE -- A literature review follows a different style, format, and structure from a research article.
| Reports on the work of others. | Reports on original research. | |
| To examine and evaluate previous literature. | To test a hypothesis and/or make an argument. May include a short literature review to introduce the subject. |
- Next: Evaluate >>
- Last Updated: Jul 30, 2024 1:42 PM
- URL: https://guides.library.jhu.edu/lit-review

The Cowles Library website will be unavailable on Tuesday, Aug. 6th from 1:00 p.m.- 5:00 p.m. due to a scheduled migration to a new platform. You can reach our list of Research Databases at https://library.drake.edu/az/databases
- Research, Study, Learning
- Archives & Special Collections

- Cowles Library
In This Section:
- Find Journal Articles
- Find Articles in Related Disciplines
- Find Streaming Video
Conducting a Literature Review
- Organizations, Associations, Societies
- For Faculty
What is a Literature Review?
Description.
A literature review, also called a review article or review of literature, surveys the existing research on a topic. The term "literature" in this context refers to published research or scholarship in a particular discipline, rather than "fiction" (like American Literature) or an individual work of literature. In general, literature reviews are most common in the sciences and social sciences.
Literature reviews may be written as standalone works, or as part of a scholarly article or research paper. In either case, the purpose of the review is to summarize and synthesize the key scholarly work that has already been done on the topic at hand. The literature review may also include some analysis and interpretation. A literature review is not a summary of every piece of scholarly research on a topic.
Why are literature reviews useful?
Literature reviews can be very helpful for newer researchers or those unfamiliar with a field by synthesizing the existing research on a given topic, providing the reader with connections and relationships among previous scholarship. Reviews can also be useful to veteran researchers by identifying potentials gaps in the research or steering future research questions toward unexplored areas. If a literature review is part of a scholarly article, it should include an explanation of how the current article adds to the conversation. (From: https://library.drake.edu/englit/criticism)
How is a literature review different from a research article?
Research articles: "are empirical articles that describe one or several related studies on a specific, quantitative, testable research question....they are typically organized into four text sections: Introduction, Methods, Results, Discussion." Source: https://psych.uw.edu/storage/writing_center/litrev.pdf)
Steps for Writing a Literature Review
1. Identify and define the topic that you will be reviewing.
The topic, which is commonly a research question (or problem) of some kind, needs to be identified and defined as clearly as possible. You need to have an idea of what you will be reviewing in order to effectively search for references and to write a coherent summary of the research on it. At this stage it can be helpful to write down a description of the research question, area, or topic that you will be reviewing, as well as to identify any keywords that you will be using to search for relevant research.
2. Conduct a Literature Search
Use a range of keywords to search databases such as PsycINFO and any others that may contain relevant articles. You should focus on peer-reviewed, scholarly articles . In SuperSearch and most databases, you may find it helpful to select the Advanced Search mode and include "literature review" or "review of the literature" in addition to your other search terms. Published books may also be helpful, but keep in mind that peer-reviewed articles are widely considered to be the “gold standard” of scientific research. Read through titles and abstracts, select and obtain articles (that is, download, copy, or print them out), and save your searches as needed. Most of the databases you will need are linked to from the Cowles Library Psychology Research guide .
3. Read through the research that you have found and take notes.
Absorb as much information as you can. Read through the articles and books that you have found, and as you do, take notes. The notes should include anything that will be helpful in advancing your own thinking about the topic and in helping you write the literature review (such as key points, ideas, or even page numbers that index key information). Some references may turn out to be more helpful than others; you may notice patterns or striking contrasts between different sources; and some sources may refer to yet other sources of potential interest. This is often the most time-consuming part of the review process. However, it is also where you get to learn about the topic in great detail. You may want to use a Citation Manager to help you keep track of the citations you have found.
4. Organize your notes and thoughts; create an outline.
At this stage, you are close to writing the review itself. However, it is often helpful to first reflect on all the reading that you have done. What patterns stand out? Do the different sources converge on a consensus? Or not? What unresolved questions still remain? You should look over your notes (it may also be helpful to reorganize them), and as you do, to think about how you will present this research in your literature review. Are you going to summarize or critically evaluate? Are you going to use a chronological or other type of organizational structure? It can also be helpful to create an outline of how your literature review will be structured.
5. Write the literature review itself and edit and revise as needed.
The final stage involves writing. When writing, keep in mind that literature reviews are generally characterized by a summary style in which prior research is described sufficiently to explain critical findings but does not include a high level of detail (if readers want to learn about all the specific details of a study, then they can look up the references that you cite and read the original articles themselves). However, the degree of emphasis that is given to individual studies may vary (more or less detail may be warranted depending on how critical or unique a given study was). After you have written a first draft, you should read it carefully and then edit and revise as needed. You may need to repeat this process more than once. It may be helpful to have another person read through your draft(s) and provide feedback.
6. Incorporate the literature review into your research paper draft. (note: this step is only if you are using the literature review to write a research paper. Many times the literature review is an end unto itself).
After the literature review is complete, you should incorporate it into your research paper (if you are writing the review as one component of a larger paper). Depending on the stage at which your paper is at, this may involve merging your literature review into a partially complete Introduction section, writing the rest of the paper around the literature review, or other processes.
These steps were taken from: https://psychology.ucsd.edu/undergraduate-program/undergraduate-resources/academic-writing-resources/writing-research-papers/writing-lit-review.html#6.-Incorporate-the-literature-r
- << Previous: Find Streaming Video
- Next: Organizations, Associations, Societies >>
- Last Updated: Aug 6, 2024 2:32 PM
Borrow & Request
Use materials placed on reserve by your instructors
Borrow books directly from other Iowa academic library partners
Borrow material from libraries around the world
Ask the library to purchase books or other research materials
Collections
Drake history and Iowa political papers
Online access to unique items from the University Archives
Books, eBooks, and videos we highlight throughout the year
Research Support
Handpicked by experts for your area of study
Schedule a one-on-one session with a librarian
A guide to the research process
Librarians who specialize in your area of study
Find, organize, and use your citations
Writing Center, Speaking Center, and other Tutoring
Tools & resources to help develop your study skills
Teaching Support
What we teach and how we can help in your courses
Connect with a librarian
Put material on reserve for your courses
Help with course material adoptions and textbook alternatives
Explore, adopt, adapt, and create open educational resources
Resources to help you publish your research
Collections & Exhibits
Research & teaching, records management, about the archives.
What we do and why
Hours, directions, and guidelines for your Archives visit
Reach, follow, and support the Archives
Guides, tutorials, and library expertise to help you succeed as a scholar
Borrowing materials, finding a study space, locating services
Library services and support directed toward Drake Online and other off-campus students
Provide feedback or resolve a problem with the library
Faculty & Staff
Resources and information literacy expertise to support your teaching
Ask a Question
Cowles Library faculty and staff profiles
What's happening at Cowles Library
Student employment at Cowles Library
Where we are and when we're open
Services for Drake alumni and visitors
Library Spaces
Navigate the library
Check availability and reserve a room
Technology in the library
Mission & Planning
Cowles Library mission and vision
Policies governing use of library resources, space, and services
Library support for diversity, equity, inclusion, and social justice

- 2507 University Avenue
- Des Moines, IA 50311
- (515) 271-2111
Trouble finding something? Try searching , or check out the Get Help page.

Research Process :: Step by Step
- Introduction
- Select Topic
- Identify Keywords
- Background Information
- Develop Research Questions
- Refine Topic
- Search Strategy
- Popular Databases
- Evaluate Sources
- Types of Periodicals
- Reading Scholarly Articles
- Primary & Secondary Sources
- Organize / Take Notes
- Writing & Grammar Resources
- Annotated Bibliography
- Literature Review
- Citation Styles
- Paraphrasing
- Privacy / Confidentiality
- Research Process
- Selecting Your Topic
- Identifying Keywords
- Gathering Background Info
- Evaluating Sources

Organize the literature review into sections that present themes or identify trends, including relevant theory. You are not trying to list all the material published, but to synthesize and evaluate it according to the guiding concept of your thesis or research question.
What is a literature review?
A literature review is an account of what has been published on a topic by accredited scholars and researchers. Occasionally you will be asked to write one as a separate assignment, but more often it is part of the introduction to an essay, research report, or thesis. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries
A literature review must do these things:
- be organized around and related directly to the thesis or research question you are developing
- synthesize results into a summary of what is and is not known
- identify areas of controversy in the literature
- formulate questions that need further research
Ask yourself questions like these:
- What is the specific thesis, problem, or research question that my literature review helps to define?
- What type of literature review am I conducting? Am I looking at issues of theory? methodology? policy? quantitative research (e.g. on the effectiveness of a new procedure)? qualitative research (e.g., studies of loneliness among migrant workers)?
- What is the scope of my literature review? What types of publications am I using (e.g., journals, books, government documents, popular media)? What discipline am I working in (e.g., nursing psychology, sociology, medicine)?
- How good was my information seeking? Has my search been wide enough to ensure I've found all the relevant material? Has it been narrow enough to exclude irrelevant material? Is the number of sources I've used appropriate for the length of my paper?
- Have I critically analyzed the literature I use? Do I follow through a set of concepts and questions, comparing items to each other in the ways they deal with them? Instead of just listing and summarizing items, do I assess them, discussing strengths and weaknesses?
- Have I cited and discussed studies contrary to my perspective?
- Will the reader find my literature review relevant, appropriate, and useful?
Ask yourself questions like these about each book or article you include:
- Has the author formulated a problem/issue?
- Is it clearly defined? Is its significance (scope, severity, relevance) clearly established?
- Could the problem have been approached more effectively from another perspective?
- What is the author's research orientation (e.g., interpretive, critical science, combination)?
- What is the author's theoretical framework (e.g., psychological, developmental, feminist)?
- What is the relationship between the theoretical and research perspectives?
- Has the author evaluated the literature relevant to the problem/issue? Does the author include literature taking positions she or he does not agree with?
- In a research study, how good are the basic components of the study design (e.g., population, intervention, outcome)? How accurate and valid are the measurements? Is the analysis of the data accurate and relevant to the research question? Are the conclusions validly based upon the data and analysis?
- In material written for a popular readership, does the author use appeals to emotion, one-sided examples, or rhetorically-charged language and tone? Is there an objective basis to the reasoning, or is the author merely "proving" what he or she already believes?
- How does the author structure the argument? Can you "deconstruct" the flow of the argument to see whether or where it breaks down logically (e.g., in establishing cause-effect relationships)?
- In what ways does this book or article contribute to our understanding of the problem under study, and in what ways is it useful for practice? What are the strengths and limitations?
- How does this book or article relate to the specific thesis or question I am developing?
Text written by Dena Taylor, Health Sciences Writing Centre, University of Toronto
http://www.writing.utoronto.ca/advice/specific-types-of-writing/literature-review
- << Previous: Annotated Bibliography
- Next: Step 5: Cite Sources >>
- Last Updated: Jun 13, 2024 4:27 PM
- URL: https://libguides.uta.edu/researchprocess
University of Texas Arlington Libraries 702 Planetarium Place · Arlington, TX 76019 · 817-272-3000
- Internet Privacy
- Accessibility
- Problems with a guide? Contact Us.

Literature Reviews
- Steps for Conducting a Lit Review
- Finding "The Literature"
- Organizing/Writing
- Peer Review
- Citation/Style Guides
Reference & Instruction Librarian

What is a Literature Review?
A literature review is not:
- just a summary of the sources
- a grouping of broad, unrelated sources
- a compilation of everything that has been written on a particular topic
- literature criticism (think English) or a book review.
So, what is it then?
A literature review :
- Surveys all of the scholarship that has been written about a particular topic (your research question).
- Provides a description , summary , and evaluation of each scholarly work.
- Synthesizes and organizes the previous research by comparing and contrasting the findings or methodology of those previous writings.
"A literature review is an account of what has been published on a topic by accredited scholars and researchers. Occasionally, you will be asked to write one as a separate assignment, ..., but more often, it is part of the introduction to an essay, research report, or thesis. In writing the literature review, you aim to convey to your reader what knowledge and ideas have been established on a topic and their strengths and weaknesses. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available or a set of summaries."
A literature review is an integrated analysis-- not just a summary-- of scholarly writings that are related directly to your research question. That is, it represents the literature that provides background information on your topic and shows a correspondence between those writings and your research question.
A literature review may be a stand-alone work or the introduction to a more extensive research paper, depending on the assignment. Rely heavily on the guidelines your instructor has given you.
Why is it important?
A literature review is important because it:
- Explains the background of research on a topic.
- Demonstrates why a topic is significant to a subject area.
- Discovers relationships between research studies/ideas.
- Identifies major themes, concepts, and researchers on a topic.
- Identifies critical gaps and points of disagreement.
- Discusses further research questions that logically come out of the previous studies.
Where Can I Find a Lit Review?
The Literature Review portion of a scholarly article is usually close to the beginning. It often follows the introduction , or may be combined with the introduction. The writer may discuss his or her research question first, or may choose to explain it while surveying previous literature.
If you are lucky, there will be a section heading that includes " literature review ". If not, look for the section of the article with the most citations or footnotes .
- Next: Steps for Conducting a Lit Review >>
- Last Updated: Aug 7, 2024 6:14 PM
- URL: https://westlibrary.txwes.edu/literaturereview
Conduct a literature review
What is a literature review.
A literature review is a summary of the published work in a field of study. This can be a section of a larger paper or article, or can be the focus of an entire paper. Literature reviews show that you have examined the breadth of knowledge and can justify your thesis or research questions. They are also valuable tools for other researchers who need to find a summary of that field of knowledge.
Unlike an annotated bibliography, which is a list of sources with short descriptions, a literature review synthesizes sources into a summary that has a thesis or statement of purpose—stated or implied—at its core.
How do I write a literature review?
Step 1: define your research scope.
- What is the specific research question that your literature review helps to define?
- Are there a maximum or minimum number of sources that your review should include?
Ask us if you have questions about refining your topic, search methods, writing tips, or citation management.
Step 2: Identify the literature
Start by searching broadly. Literature for your review will typically be acquired through scholarly books, journal articles, and/or dissertations. Develop an understanding of what is out there, what terms are accurate and helpful, etc., and keep track of all of it with citation management tools . If you need help figuring out key terms and where to search, ask us .
Use citation searching to track how scholars interact with, and build upon, previous research:
- Mine the references cited section of each relevant source for additional key sources
- Use Google Scholar or Scopus to find other sources that have cited a particular work
Step 3: Critically analyze the literature
Key to your literature review is a critical analysis of the literature collected around your topic. The analysis will explore relationships, major themes, and any critical gaps in the research expressed in the work. Read and summarize each source with an eye toward analyzing authority, currency, coverage, methodology, and relationship to other works. The University of Toronto's Writing Center provides a comprehensive list of questions you can use to analyze your sources.
Step 4: Categorize your resources
Divide the available resources that pertain to your research into categories reflecting their roles in addressing your research question. Possible ways to categorize resources include organization by:
- methodology
- theoretical/philosophical approach
Regardless of the division, each category should be accompanied by thorough discussions and explanations of strengths and weaknesses, value to the overall survey, and comparisons with similar sources. You may have enough resources when:
- You've used multiple databases and other resources (web portals, repositories, etc.) to get a variety of perspectives on the research topic.
- The same citations are showing up in a variety of databases.
Additional resources
Undergraduate student resources.
- Literature Review Handout (University of North Carolina at Chapel Hill)
- Learn how to write a review of literature (University of Wisconsin-Madison)
Graduate student and faculty resources
- Information Research Strategies (University of Arizona)
- Literature Reviews: An Overview for Graduate Students (NC State University)
- Oliver, P. (2012). Succeeding with Your Literature Review: A Handbook for Students [ebook]
- Machi, L. A. & McEvoy, B. T. (2016). The Literature Review: Six Steps to Success [ebook]
- Graustein, J. S. (2012). How to Write an Exceptional Thesis or Dissertation: A Step-by-Step Guide from Proposal to Successful Defense [ebook]
- Thomas, R. M. & Brubaker, D. L. (2008). Theses and Dissertations: A Guide to Planning, Research, and Writing

Research Process Guide
- Step 1 - Identifying and Developing a Topic
- Step 2 - Narrowing Your Topic
- Step 3 - Developing Research Questions
- Step 4 - Conducting a Literature Review
- Step 5 - Choosing a Conceptual or Theoretical Framework
- Step 6 - Determining Research Methodology
- Step 6a - Determining Research Methodology - Quantitative Research Methods
- Step 6b - Determining Research Methodology - Qualitative Design
- Step 7 - Considering Ethical Issues in Research with Human Subjects - Institutional Review Board (IRB)
- Step 8 - Collecting Data
- Step 9 - Analyzing Data
- Step 10 - Interpreting Results
- Step 11 - Writing Up Results
Step 4: Conducting a Literature Review

In order to understand your topic, before you conduct your research, it is extremely important to immerse yourself in the research that has been done on your topic and the topics that might be adjacent to your particular research interest or questions. “a researcher cannot perform significant research without first understanding the literature in the field” (Boote & Beile, 2005, p. 3). Essentially, When writing a thesis or research proposal, the review of the literature would be your Chapter 2 .
Frankly, the literature review is often the first major challenge of the writing process. Sometimes, the task to review and synthesize all of the previous research on and around your topic can feel overwhelming. Although the literature review is foundational to situate your research within the body of literature on your topic, there is almost no literature on the challenges and pitfalls of writing a literature review (Randolph, 2009).
Boote and Beile (2005) reveal through their research on dissertation writing, that although a sophisticated literature review is essential for substantial research, they are often poorly written and lack organizational structure and conceptual relevance. So, the question is, how can you write a literature that is well-organized, comprehensive and situate your research within the literature?
Onweugbuzie et al. (2012) identify 23 core components of an effective literature review in their research and are referred to as the standard checklist for most empirical researchers. The list includes the following:
- What has been researched and what needs to be in the future.
- Identify variables within the literature that are relevant to your study.
- Identify the relationship between theory and practice within the literature.
- Discuss the quality of research with particular emphasis on the exemplary studies.
- Examine the methodologies and research design used throughout the literature, and evaluate the efficacy.
- Pay attention to any contradictions within the literature and
- Make sure to not replicate studies that have already been completed, however, if there are similarities, identify how your study and variables examined are important, different, and relevant.
How to complete a literature review
Fair Warning: The literature review is often time-consuming and can feel like an endless process. Don’t Give Up! It is the first major hurdle of the research proposal process. Once you have completed the literature review, you will have a good idea of what is significant, relevant and novel about your research. The key is to spend time reading, recording important findings, and organizing the scholarly literature on (and around) your topic.
At this point, it is important to distinguish between scholarly literature and other sources. You need to keep in mind that you are only reviewing scholarly literature, which includes sources and studies that have a clear methodology, empirical evidence, results and conclusions. These studies are PEER-REVIEWED, meaning, contemporaries in the field have reviewed the research methods and findings of the literature, and found them relevant, significant, authentic and valid (Wakefield, 2015).
Where do you begin? Great question.
According to Randolph (2009), the goal of a literature review is to integrate and generalize findings across studies, debate findings within a field, resolve the debate, and discuss the language specific to the field. For a meta-analysis, which is a common strategy for a literature review, the goal is largely to integrate quantitative findings across the research on the topic. For other strategies to complete the literature review, the goals may be to critically analyze previous studies, identify central themes or issues within the existing literature or analyze an argument in the field (Randolph, 2009). In literature reviews for dissertation, the goal is to largely interrogate and analyze the current findings to find weaknesses or contradictions in order to place your study within the context of the current literature and to justify your study’s relevance. So in essence, the goals of literature review, regardless of the strategy, are not only to deal with the central theme across the literature and to present a thematic analysis of that literature, but also, and perhaps more importantly, it is to focus on whether the body of knowledge is credible, reliable and valid based on the methodological approaches and outcomes of the literature in the field (Wakefield, 2015).
A few initial steps are:
- Develop a list of key words and phrases that relate to your topic and questions (Denney & Tewksbury, 2013).
- Search for relevant sources using useful databases found in Kean University’s Library :
- Kean University’s WorldCat Discovery single-search application
- ERIC (education)
- JSTOR (multidisciplinary)
- Project Muse (humanities and social science)
- Web of Science (citation searching)
- Google Scholar
- Set up an account with a bibliographic citation manager like EndNote Online (access provided by Kean University) or a freely available option such as Zotero. The EndNote Online Guide provides separate on-campus and off-campus account registration instructions. A bibliographic citation manager will not only help you manage and organize your sources, but it will also help you format your references in various citation styles.
- Take advantage of research support options provided by Kean’s librarians, including workshops , appointments with a librarian , and 24/7 Chat .
|
Think about adding “Kean University” to your library link within your . You will be able to gain full access to “full text” articles while searching in Google Scholar. |
If you identify a source (article or book) that is not available through Kean University’s library collections, you may submit an Interlibrary Loan request. Book or article records found in the WorldCat Discovery database will feature an Interlibrary Loan request option. However, you may also utilize the Interlibrary Loan form .
You may also use the VALE Reciprocal Borrowing Program , which enables Kean University students and faculty to check out books from libraries at other New Jersey colleges and universities. To participate in this program, a researcher must first obtain a signed "VALE Reciprocal Borrowing Application Form" from the Nancy Thompson Learning Commons before they can borrow at one of the participating libraries .
What are the sources that are appropriate for a literature review? According to Garrard (2009) and others scientific or empirical research refers to the:
…theoretical and research publications in scientific journals, reference books, government practice, policy statements, and other materials about the theory, practice, and results of scientific inquiry. These materials and publications are produced by individuals or groups in universities, foundations, government research laboratories, and other nonprofit or for-profit organizations (p.4). Onwuegbuzie et al. (2010; as cited in Onwuegbuzie et al., 2012) goes further and describes the literature that could be included in a literature review, “research articles,… essays, article reviews, monographs, dissertations, books, Internet websites, video, interview transcripts, encyclopedias, company reports, trade catalogues, government documents, congressional/parliamentary bills…” (p. 7). However, Onwuegbuzie et al. (2012) builds on this definition, by saying that a literature review is largely, “a systematic, explicit, and reproducible method for identifying, evaluating, and synthesizing the existing body of completed and recorded work produced by researchers, scholars and practitioners” (p.3).
So, what does this mean for you, the researcher and author of the literature review? You want to use multiple source types. Additionally, stick to the parameters laid out by Onwuegbuzie et al. (2012) in that the literature reviewed should be an evaluation and synthesis of the existing work completed by “researchers, scholars and practitioners.” The list of sources should be semi-exhaustive and representative of the field.
Next, you should:
3. Evaluate and select your sources. Read the abstract first to see if the source is relevant to your topic (Wakefield, 2015; Denney & Tewksbury, 2013; Randolph, 2009).
- Is this source peer-reviewed?
- Is this source presenting empirical evidence, meeting the threshold for scholarly research?
- Is this topic relevant to my research topic/questions?
| While reading your sources, take notes and keep track of what each article says: |
| Creating a is a helpful way to keep track of your sources. Including title, author, topic(s), methods and findings, as well as direct quotes that you think might be meaningful to your literature review would be helpful. Also, it would be important to note how you retrieved your source so that, theoretically, other researchers could replicate your literature review (Randolph, 2009). |
Organizing your Literature Review:
Outline your literature review- how do you want it organized? You are “synthesizing” the literature as your purpose here. What structure works best for your topic and study? The most common formats are (Randolph, 2009; Onwuegbuzie et al., 2012):
- Historical format - literature is reviewed chronologically.This method is preferred when there is a goal of analyzing the progression of research methods, theories or practices over time.
- Conceptual format - centered arounds the propositions in research rationale or a theoretical- centered review which is organized according to the theories in the literature.
- Methodological format - this involves the discussion of methodology as in an imperial paper including an introduction, method, results and discussion. This approach is most commonly used in meta- analytical reports.
| Be sure to structure your literature review so it makes sense to you. You can organize it thematically, chronologically, methodically or any other way that works for you and your understanding of the topic. |
Let’s talk about synthesis.
A literature review is not only a review of the empirical research, but it is also evaluation and synthesis of the research. Boote and Beile (2005) have created a five- category list for evaluating a literature review. The categories are coverage, synthesis, methodology, significance, and rhetoric .
- You need to create a justified criteria for including and excluding studies from your review
- You need to discuss what has been done in the field and what still needs to be done.
- Place the topic or problem within the greater context of scholarly literature.
- Place the topic or problem within the historical context.
- Discuss the subject vocabulary.
- Articulate the important variables and phenomena that are relevant to the topic.
- Synthesize and discuss a new perspective on the literature.
- Identify the main methods and research techniques that have been used in the field as well as their advantages/disadvantages.
- Relate ideas and theories to research methodologies.
- Rationalize the practical significance of the research problem.
- Rationalize the scholarly significance of the research problem.
- Write in coherent language and be sure the organization/ structure of the review makes sense.
Synthesis is difficult - you need to articulate what this literature means for your research and/or how does the literature inform the purpose, impact, methodology of your study? Rather than summarizing, the idea behind synthesis is taking the information you have discussed and drawing your own conclusions, making connections between the literature and your study.
Boote, D. N., & Beile, P. (2005). Scholars before researchers: On the centrality of the dissertation literature review in research preparation. Educational Researcher, 34 (6), 3-15. https://www.jstor.org/stable/3699805
Denney, A. S., & Tewksbury, R. (2013). How to write a literature review. Journal of Criminal Justice Education, 24 (2), 218-234. https://doi-org.kean.idm.oclc.org/10.1080/10511253.2012.730617
Garrard, J. (2009). Health sciences literature review made easy: The matrix method. Jones and Bartlett.
Onwuegbuzie, A. J., Leech, N. L., & Collins, K. M. (2012). Qualitative analysis techniques for the review of the literature. Qualitative Report, 17( 28), 1-28.
Randolph, J. (2009). A guide to writing the dissertation literature review. Practical Assessment, Research, and Evaluation, 14 (1), 13.
Wakefield, A. (2015). Synthesising the literature as part of a literature review. Nursing Standard, 29 (29), 44-51. https://doi.org/10.7748/ns.29.29.44.e8957
- Last Updated: Jun 29, 2023 1:35 PM
- URL: https://libguides.kean.edu/ResearchProcessGuide
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Comput Biol
- v.9(7); 2013 Jul

Ten Simple Rules for Writing a Literature Review
Marco pautasso.
1 Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France
2 Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,

The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
Funding Statement
This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.


What is a Literature Review? How to Write It (with Examples)

A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship, demonstrating your understanding of the topic and showing how your work contributes to the ongoing conversation in the field. Learning how to write a literature review is a critical tool for successful research. Your ability to summarize and synthesize prior research pertaining to a certain topic demonstrates your grasp on the topic of study, and assists in the learning process.
Table of Contents
- What is the purpose of literature review?
- a. Habitat Loss and Species Extinction:
- b. Range Shifts and Phenological Changes:
- c. Ocean Acidification and Coral Reefs:
- d. Adaptive Strategies and Conservation Efforts:
How to write a good literature review
- Choose a Topic and Define the Research Question:
- Decide on the Scope of Your Review:
- Select Databases for Searches:
- Conduct Searches and Keep Track:
- Review the Literature:
- Organize and Write Your Literature Review:
- How to write a literature review faster with Paperpal?
- Frequently asked questions
What is a literature review?
A well-conducted literature review demonstrates the researcher’s familiarity with the existing literature, establishes the context for their own research, and contributes to scholarly conversations on the topic. One of the purposes of a literature review is also to help researchers avoid duplicating previous work and ensure that their research is informed by and builds upon the existing body of knowledge.

What is the purpose of literature review?
A literature review serves several important purposes within academic and research contexts. Here are some key objectives and functions of a literature review: 2
1. Contextualizing the Research Problem: The literature review provides a background and context for the research problem under investigation. It helps to situate the study within the existing body of knowledge.
2. Identifying Gaps in Knowledge: By identifying gaps, contradictions, or areas requiring further research, the researcher can shape the research question and justify the significance of the study. This is crucial for ensuring that the new research contributes something novel to the field.
Find academic papers related to your research topic faster. Try Research on Paperpal
3. Understanding Theoretical and Conceptual Frameworks: Literature reviews help researchers gain an understanding of the theoretical and conceptual frameworks used in previous studies. This aids in the development of a theoretical framework for the current research.
4. Providing Methodological Insights: Another purpose of literature reviews is that it allows researchers to learn about the methodologies employed in previous studies. This can help in choosing appropriate research methods for the current study and avoiding pitfalls that others may have encountered.
5. Establishing Credibility: A well-conducted literature review demonstrates the researcher’s familiarity with existing scholarship, establishing their credibility and expertise in the field. It also helps in building a solid foundation for the new research.
6. Informing Hypotheses or Research Questions: The literature review guides the formulation of hypotheses or research questions by highlighting relevant findings and areas of uncertainty in existing literature.
Literature review example
Let’s delve deeper with a literature review example: Let’s say your literature review is about the impact of climate change on biodiversity. You might format your literature review into sections such as the effects of climate change on habitat loss and species extinction, phenological changes, and marine biodiversity. Each section would then summarize and analyze relevant studies in those areas, highlighting key findings and identifying gaps in the research. The review would conclude by emphasizing the need for further research on specific aspects of the relationship between climate change and biodiversity. The following literature review template provides a glimpse into the recommended literature review structure and content, demonstrating how research findings are organized around specific themes within a broader topic.
Literature Review on Climate Change Impacts on Biodiversity:
Climate change is a global phenomenon with far-reaching consequences, including significant impacts on biodiversity. This literature review synthesizes key findings from various studies:
a. Habitat Loss and Species Extinction:
Climate change-induced alterations in temperature and precipitation patterns contribute to habitat loss, affecting numerous species (Thomas et al., 2004). The review discusses how these changes increase the risk of extinction, particularly for species with specific habitat requirements.
b. Range Shifts and Phenological Changes:
Observations of range shifts and changes in the timing of biological events (phenology) are documented in response to changing climatic conditions (Parmesan & Yohe, 2003). These shifts affect ecosystems and may lead to mismatches between species and their resources.
c. Ocean Acidification and Coral Reefs:
The review explores the impact of climate change on marine biodiversity, emphasizing ocean acidification’s threat to coral reefs (Hoegh-Guldberg et al., 2007). Changes in pH levels negatively affect coral calcification, disrupting the delicate balance of marine ecosystems.
d. Adaptive Strategies and Conservation Efforts:
Recognizing the urgency of the situation, the literature review discusses various adaptive strategies adopted by species and conservation efforts aimed at mitigating the impacts of climate change on biodiversity (Hannah et al., 2007). It emphasizes the importance of interdisciplinary approaches for effective conservation planning.

Strengthen your literature review with factual insights. Try Research on Paperpal for free!
Writing a literature review involves summarizing and synthesizing existing research on a particular topic. A good literature review format should include the following elements.
Introduction: The introduction sets the stage for your literature review, providing context and introducing the main focus of your review.
- Opening Statement: Begin with a general statement about the broader topic and its significance in the field.
- Scope and Purpose: Clearly define the scope of your literature review. Explain the specific research question or objective you aim to address.
- Organizational Framework: Briefly outline the structure of your literature review, indicating how you will categorize and discuss the existing research.
- Significance of the Study: Highlight why your literature review is important and how it contributes to the understanding of the chosen topic.
- Thesis Statement: Conclude the introduction with a concise thesis statement that outlines the main argument or perspective you will develop in the body of the literature review.
Body: The body of the literature review is where you provide a comprehensive analysis of existing literature, grouping studies based on themes, methodologies, or other relevant criteria.
- Organize by Theme or Concept: Group studies that share common themes, concepts, or methodologies. Discuss each theme or concept in detail, summarizing key findings and identifying gaps or areas of disagreement.
- Critical Analysis: Evaluate the strengths and weaknesses of each study. Discuss the methodologies used, the quality of evidence, and the overall contribution of each work to the understanding of the topic.
- Synthesis of Findings: Synthesize the information from different studies to highlight trends, patterns, or areas of consensus in the literature.
- Identification of Gaps: Discuss any gaps or limitations in the existing research and explain how your review contributes to filling these gaps.
- Transition between Sections: Provide smooth transitions between different themes or concepts to maintain the flow of your literature review.
Write and Cite as you go with Paperpal Research. Start now for free.
Conclusion: The conclusion of your literature review should summarize the main findings, highlight the contributions of the review, and suggest avenues for future research.
- Summary of Key Findings: Recap the main findings from the literature and restate how they contribute to your research question or objective.
- Contributions to the Field: Discuss the overall contribution of your literature review to the existing knowledge in the field.
- Implications and Applications: Explore the practical implications of the findings and suggest how they might impact future research or practice.
- Recommendations for Future Research: Identify areas that require further investigation and propose potential directions for future research in the field.
- Final Thoughts: Conclude with a final reflection on the importance of your literature review and its relevance to the broader academic community.

Conducting a literature review
Conducting a literature review is an essential step in research that involves reviewing and analyzing existing literature on a specific topic. It’s important to know how to do a literature review effectively, so here are the steps to follow: 1
Choose a Topic and Define the Research Question:
- Select a topic that is relevant to your field of study.
- Clearly define your research question or objective. Determine what specific aspect of the topic do you want to explore?
Decide on the Scope of Your Review:
- Determine the timeframe for your literature review. Are you focusing on recent developments, or do you want a historical overview?
- Consider the geographical scope. Is your review global, or are you focusing on a specific region?
- Define the inclusion and exclusion criteria. What types of sources will you include? Are there specific types of studies or publications you will exclude?
Select Databases for Searches:
- Identify relevant databases for your field. Examples include PubMed, IEEE Xplore, Scopus, Web of Science, and Google Scholar.
- Consider searching in library catalogs, institutional repositories, and specialized databases related to your topic.
Conduct Searches and Keep Track:
- Develop a systematic search strategy using keywords, Boolean operators (AND, OR, NOT), and other search techniques.
- Record and document your search strategy for transparency and replicability.
- Keep track of the articles, including publication details, abstracts, and links. Use citation management tools like EndNote, Zotero, or Mendeley to organize your references.
Review the Literature:
- Evaluate the relevance and quality of each source. Consider the methodology, sample size, and results of studies.
- Organize the literature by themes or key concepts. Identify patterns, trends, and gaps in the existing research.
- Summarize key findings and arguments from each source. Compare and contrast different perspectives.
- Identify areas where there is a consensus in the literature and where there are conflicting opinions.
- Provide critical analysis and synthesis of the literature. What are the strengths and weaknesses of existing research?
Organize and Write Your Literature Review:
- Literature review outline should be based on themes, chronological order, or methodological approaches.
- Write a clear and coherent narrative that synthesizes the information gathered.
- Use proper citations for each source and ensure consistency in your citation style (APA, MLA, Chicago, etc.).
- Conclude your literature review by summarizing key findings, identifying gaps, and suggesting areas for future research.
Whether you’re exploring a new research field or finding new angles to develop an existing topic, sifting through hundreds of papers can take more time than you have to spare. But what if you could find science-backed insights with verified citations in seconds? That’s the power of Paperpal’s new Research feature!
How to write a literature review faster with Paperpal?
Paperpal, an AI writing assistant, integrates powerful academic search capabilities within its writing platform. With the Research feature, you get 100% factual insights, with citations backed by 250M+ verified research articles, directly within your writing interface with the option to save relevant references in your Citation Library. By eliminating the need to switch tabs to find answers to all your research questions, Paperpal saves time and helps you stay focused on your writing.
Here’s how to use the Research feature:
- Ask a question: Get started with a new document on paperpal.com. Click on the “Research” feature and type your question in plain English. Paperpal will scour over 250 million research articles, including conference papers and preprints, to provide you with accurate insights and citations.
- Review and Save: Paperpal summarizes the information, while citing sources and listing relevant reads. You can quickly scan the results to identify relevant references and save these directly to your built-in citations library for later access.
- Cite with Confidence: Paperpal makes it easy to incorporate relevant citations and references into your writing, ensuring your arguments are well-supported by credible sources. This translates to a polished, well-researched literature review.
The literature review sample and detailed advice on writing and conducting a review will help you produce a well-structured report. But remember that a good literature review is an ongoing process, and it may be necessary to revisit and update it as your research progresses. By combining effortless research with an easy citation process, Paperpal Research streamlines the literature review process and empowers you to write faster and with more confidence. Try Paperpal Research now and see for yourself.
Frequently asked questions
A literature review is a critical and comprehensive analysis of existing literature (published and unpublished works) on a specific topic or research question and provides a synthesis of the current state of knowledge in a particular field. A well-conducted literature review is crucial for researchers to build upon existing knowledge, avoid duplication of efforts, and contribute to the advancement of their field. It also helps researchers situate their work within a broader context and facilitates the development of a sound theoretical and conceptual framework for their studies.
Literature review is a crucial component of research writing, providing a solid background for a research paper’s investigation. The aim is to keep professionals up to date by providing an understanding of ongoing developments within a specific field, including research methods, and experimental techniques used in that field, and present that knowledge in the form of a written report. Also, the depth and breadth of the literature review emphasizes the credibility of the scholar in his or her field.
Before writing a literature review, it’s essential to undertake several preparatory steps to ensure that your review is well-researched, organized, and focused. This includes choosing a topic of general interest to you and doing exploratory research on that topic, writing an annotated bibliography, and noting major points, especially those that relate to the position you have taken on the topic.
Literature reviews and academic research papers are essential components of scholarly work but serve different purposes within the academic realm. 3 A literature review aims to provide a foundation for understanding the current state of research on a particular topic, identify gaps or controversies, and lay the groundwork for future research. Therefore, it draws heavily from existing academic sources, including books, journal articles, and other scholarly publications. In contrast, an academic research paper aims to present new knowledge, contribute to the academic discourse, and advance the understanding of a specific research question. Therefore, it involves a mix of existing literature (in the introduction and literature review sections) and original data or findings obtained through research methods.
Literature reviews are essential components of academic and research papers, and various strategies can be employed to conduct them effectively. If you want to know how to write a literature review for a research paper, here are four common approaches that are often used by researchers. Chronological Review: This strategy involves organizing the literature based on the chronological order of publication. It helps to trace the development of a topic over time, showing how ideas, theories, and research have evolved. Thematic Review: Thematic reviews focus on identifying and analyzing themes or topics that cut across different studies. Instead of organizing the literature chronologically, it is grouped by key themes or concepts, allowing for a comprehensive exploration of various aspects of the topic. Methodological Review: This strategy involves organizing the literature based on the research methods employed in different studies. It helps to highlight the strengths and weaknesses of various methodologies and allows the reader to evaluate the reliability and validity of the research findings. Theoretical Review: A theoretical review examines the literature based on the theoretical frameworks used in different studies. This approach helps to identify the key theories that have been applied to the topic and assess their contributions to the understanding of the subject. It’s important to note that these strategies are not mutually exclusive, and a literature review may combine elements of more than one approach. The choice of strategy depends on the research question, the nature of the literature available, and the goals of the review. Additionally, other strategies, such as integrative reviews or systematic reviews, may be employed depending on the specific requirements of the research.
The literature review format can vary depending on the specific publication guidelines. However, there are some common elements and structures that are often followed. Here is a general guideline for the format of a literature review: Introduction: Provide an overview of the topic. Define the scope and purpose of the literature review. State the research question or objective. Body: Organize the literature by themes, concepts, or chronology. Critically analyze and evaluate each source. Discuss the strengths and weaknesses of the studies. Highlight any methodological limitations or biases. Identify patterns, connections, or contradictions in the existing research. Conclusion: Summarize the key points discussed in the literature review. Highlight the research gap. Address the research question or objective stated in the introduction. Highlight the contributions of the review and suggest directions for future research.
Both annotated bibliographies and literature reviews involve the examination of scholarly sources. While annotated bibliographies focus on individual sources with brief annotations, literature reviews provide a more in-depth, integrated, and comprehensive analysis of existing literature on a specific topic. The key differences are as follows:
| Annotated Bibliography | Literature Review | |
| Purpose | List of citations of books, articles, and other sources with a brief description (annotation) of each source. | Comprehensive and critical analysis of existing literature on a specific topic. |
| Focus | Summary and evaluation of each source, including its relevance, methodology, and key findings. | Provides an overview of the current state of knowledge on a particular subject and identifies gaps, trends, and patterns in existing literature. |
| Structure | Each citation is followed by a concise paragraph (annotation) that describes the source’s content, methodology, and its contribution to the topic. | The literature review is organized thematically or chronologically and involves a synthesis of the findings from different sources to build a narrative or argument. |
| Length | Typically 100-200 words | Length of literature review ranges from a few pages to several chapters |
| Independence | Each source is treated separately, with less emphasis on synthesizing the information across sources. | The writer synthesizes information from multiple sources to present a cohesive overview of the topic. |
References
- Denney, A. S., & Tewksbury, R. (2013). How to write a literature review. Journal of criminal justice education , 24 (2), 218-234.
- Pan, M. L. (2016). Preparing literature reviews: Qualitative and quantitative approaches . Taylor & Francis.
- Cantero, C. (2019). How to write a literature review. San José State University Writing Center .
Paperpal is an AI writing assistant that help academics write better, faster with real-time suggestions for in-depth language and grammar correction. Trained on millions of research manuscripts enhanced by professional academic editors, Paperpal delivers human precision at machine speed.
Try it for free or upgrade to Paperpal Prime , which unlocks unlimited access to premium features like academic translation, paraphrasing, contextual synonyms, consistency checks and more. It’s like always having a professional academic editor by your side! Go beyond limitations and experience the future of academic writing. Get Paperpal Prime now at just US$19 a month!
Related Reads:
- Empirical Research: A Comprehensive Guide for Academics
- How to Write a Scientific Paper in 10 Steps
- How Long Should a Chapter Be?
- How to Use Paperpal to Generate Emails & Cover Letters?
6 Tips for Post-Doc Researchers to Take Their Career to the Next Level
Self-plagiarism in research: what it is and how to avoid it, you may also like, the ai revolution: authors’ role in upholding academic..., the future of academia: how ai tools are..., how to write a research proposal: (with examples..., how to write your research paper in apa..., how to choose a dissertation topic, how to write a phd research proposal, how to write an academic paragraph (step-by-step guide), five things authors need to know when using..., 7 best referencing tools and citation management software..., maintaining academic integrity with paperpal’s generative ai writing....

Graduate Research: Guide to the Literature Review
- "Literature review" defined
- Research Communication Graphic
- Literature Review Steps
- Search techniques
- Finding Additional "Items
- Evaluating information
- Citing Styles
- Ethical Use of Information
- Research Databases This link opens in a new window
- Get Full Text
- Reading a Scholarly Article
- Author Rights
- Selecting a publisher
Introduction to Research Process: Literature Review Steps
When seeking information for a literature review or for any purpose, it helps to understand information-seeking as a process that you can follow. 5 Each of the six (6) steps has its own section in this web page with more detail. Do (and re-do) the following six steps:
1. Define your topic. The first step is defining your task -- choosing a topic and noting the questions you have about the topic. This will provide a focus that guides your strategy in step II and will provide potential words to use in searches in step III.
2. Develop a strategy. Strategy involves figuring out where the information might be and identifying the best tools for finding those types of sources. The strategy section identifies specific types of research databases to use for specific purposes.
3. Locate the information . In this step, you implement the strategy developed in II in order to actually locate specific articles, books, technical reports, etc.
4. Use and Evaluate the information. Having located relevant and useful material, in step IV you read and analyze the items to determine whether they have value for your project and credibility as sources.
5. Synthesize. In step V, you will make sense of what you've learned and demonstrate your knowledge. You will thoroughly understand, organize and integrate the information --become knowledgeable-- so that you are able to use your own words to support and explain your research project and its relationship to existing research by others.
6. Evaluate your work. At every step along the way, you should evaluate your work. However, this final step is a last check to make sure your work is complete and of high quality.
Continue below to begin working through the process.
5. Eisenberg, M. B., & Berkowitz, R. E. (1990). Information Problem-Solving: the Big Six Skills Approach to Library & Information Skills Instruction . Norwood, NJ: Ablex Publishing.
1. Define your topic.
I. Define your topic
A. Many students have difficulty selecting a topic. You want to find a topic you find interesting and will enjoy learning more about.
B. Students often select a topic that is too broad. You may have a broad topic in mind initially and will need to narrow it.
1. To help narrow a broad topic :
a. Brainstorm.
1). Try this technique for brainstorming to narrow your focus.
a) Step 1. Write down your broad topic.
b) Step 2. Write down a "specific kind" or "specific aspect" of the topic you identified in step 1.
c) Step 3. Write down an aspect --such as an attribute or behavior-- of the "specific kind" you identified in step 2.
d) Step 4. Continue to add levels of specificity as needed to get to a focus that is manageable. However, you may want to begin researching the literature before narrowing further to give yourself the opportunity to explore what others are doing and how that might impact the direction that you take for your own research.
2) Three examples of using the narrowing technique. These examples start with very, very broad topics, so the topic at step 3 or 4 in these examples would be used for a preliminary search in the literature in order to identify a more specific focus. Greater specificity than level 3 or 4 will ultimately be necessary for developing a specific research question. And we may discover in our preliminary research that we need to alter the direction that we originally were taking.
a) Example 1.
Step 1. information security
Step 2. protocols
Step 3. handshake protocol
Brainstorming has brought us to focus on the handshake protocol.
b) Example 2.
Step 1. information security
Step 2. single sign-on authentication
Step 3. analyzing
Step 4. methods
Brainstorming has brought us to focus on methods for analyzing the security of single sign-on authentication
c) Example 3. The diagram below is an example using the broad topic of "software" to show two potential ways to begin to narrow the topic.
C. Once you have completed the brainstorming process and your topic is more focused, you can do preliminary research to help you identify a specific research question .
1) Examine overview sources such as subject-specific encyclopedias and textbooks that are likely to break down your specific topic into sub-topics and to highlight core issues that could serve as possible research questions. [See section II. below on developing a strategy to learn how to find these encyclopedias]
2). Search the broad topic in a research database that includes scholarly journals and professional magazines (to find technical and scholarly articles) and scan recent article titles for ideas. [See section II. below on developing a strategy to learn how to find trade and scholarly journal articles]
D. Once you have identified a research question or questions, ask yourself what you need to know to answer the questions. For example,
1. What new knowledge do I need to gain?
2. What has already been answered by prior research of other scholars?
E. Use the answers to the questions in C. to identify what words to use to describe the topic when you are doing searches.
1. Identify key words
a. For example , if you are investigating "security audits in banking", key terms to combine in your searches would be: security, audits, banking.
2. Create a list of alternative ways of referring to a key word or phrase
a.For example , "information assurance" may be referred to in various ways such as: "information assurance," "information security," and "computer security."
b. Use these alternatives when doing searches.
3. As you are searching, pay attention to how others are writing about the topic and add new words or phrases to your searches if appropriate.
2. Develop a strategy.
II. Develop a strategy for finding the information.
A. Start by considering what types of source might contain the information you need . Do you need a dictionary for definitions? a directory for an address? the history of a concept or technique that might be in a book or specialized encyclopedia? today's tech news in an online tech magazine or newspaper? current research in a journal article? background information that might be in a specialized encyclopedia? data or statistics from a specific organization or website? Note that you will typically have online access to these source types.
B. This section provides a description of some of the common types of information needed for research.
1. For technical and business analysis , look for articles in technical and trade magazines . These articles are written by information technology professionals to help other IT professionals do their jobs better. Content might include news on new developments in hardware or software, techniques, tools, and practical advice. Technical journals are also likely to have product ads relevant to information technology workers and to have job ads. Examples iof technical magazines include Network Computing and IEEE Spectrum .
2. To read original research studies , look for articles in scholarly journals and conference proceedings . They will provide articles written by information technology professionals who are reporting original research; that is, research that has been done by the authors and is being reported for the first time. The audience for original research articles is other information technology scholars and professionals. Examples of scholarly journals include Journal of Applied Security Research , Journal of Management Information Systems , IEEE Transactions on Computers , and ACM Transactions on Information and System Security .
3. For original research being reported to funding agencies , look for technical reports on agency websites. Technical reports are researcher reports to funding agencies about progress on or completion of research funded by the agency.
4. For in-depth, comprehensive information on a topic , look for book-length volumes . All chapters in the book might be written by the same author(s) or might be a collection of separate papers written by different authors.
5. To learn about an unfamiliar topic , use textbooks , specialized encyclopedias and handbooks to get get overviews of topics, history/background, and key issues explained.
6. For instructions for hardware, software, networking, etc., look for manuals that provide step-by-step instructions.
7. For technical details about inventions (devices, instruments, machines), look for patent documents .
C. NOTE - In order to search for and find original research studies, it will help if you understand how information is produced, packaged and communicated within your profession. This is explained in the tab "Research Communication: Graphic."
3. Locate the information.
III. Locate the information
A. Use search tools designed to find the sources you want. Types of sources were described in section II. above.
Always feel free to Ask a librarian for assistance when you have questions about where and how locate the information you need.
B. Evaluate the search results (no matter where you find the information)
1. Evaluate the items you find using at least these 5 criteria:
a. accuracy -- is the information reliable and error free?
1) Is there an editor or someone who verifies/checks the information?
2) Is there adequate documentation: bibliography, footnotes, credits?
3) Are the conclusions justified by the information presented?
b. authority -- is the source of the information reputable?
1) How did you find the source of information: an index to edited/peer-reviewed material, in a bibliography from a published article, etc.?
2) What type of source is it: sensationalistic, popular, scholarly?
c. objectivity -- does the information show bias?
1) What is the purpose of the information: to inform, persuade, explain, sway opinion, advertise?
2) Does the source show political or cultural biases?
d. currency -- is the information current? does it cover the time period you need?
e. coverage -- does it provide the evidence or information you need?
2. Is the search producing the material you need? -- the right content? the right quality? right time period? right geographical location? etc. If not, are you using
a. the right sources?
b. the right tools to get to the sources?
c. are you using the right words to describe the topic?
3. Have you discovered additional terms that should be searched? If so, search those terms.
4. Have you discovered additional questions you need to answer? If so, return to section A above to begin to answer new questions.
4. Use and evaluate the information.
IV. Use the information.
A. Read, hear or view the source
1. Evaluate: Does the material answer your question(s)? -- right content? If not, return to B.
2. Evaluate: Is the material appropriate? -- right quality? If not, return to B.
B. Extract the information from the source : copy/download information, take notes, record citation, keep track of items using a citation manager.
1. Note taking (these steps will help you when you begin to write your thesis and/or document your project.):
a. Write the keywords you use in your searches to avoid duplicating previous searches if you return to search a research database again. Keeping track of keywords used will also save you time if your search is interrupted or you need return and do the search again for some other reason. It will help you remember which search terms worked successfully in which databases
b. Write the citations or record the information needed to cite each article/document you plan to read and use, or make sure that any saved a copy of the article includes all the information needed to cite it. Some article pdf files may not include all of the information needed to cite, and it's a waste of your valuable time to have to go back to search and find the items again in order to be able to cite them. Using citation management software such as EndNote will help keep track of citations and help create bibliographies for your research papers.
c. Write a summary of each article you read and/or why you want to use it.
5. Synthesize.
V. Synthesize.
A. Organize and integrate information from multiple sources
B. Present the information (create report, speech, etc. that communicates)
C. Cite material using the style required by your professor or by the venue (conference, publication, etc.). For help with citation styles, see Guide to Citing Sources . A link to the citing guide is also available in the "Get Help" section on the left side of the Library home page
6. Evaluate your work.
VI. Evaluate the paper, speech, or whatever you are using to communicate your research.
A. Is it effective?
B. Does it meet the requirements?
C. Ask another student or colleague to provide constructive criticism of your paper/project.
- << Previous: Research Communication Graphic
- Next: Search techniques >>
- Last Updated: Apr 15, 2024 3:27 PM
- URL: https://library.dsu.edu/graduate-research
Doing a Scoping Review: A Practical, Step-by-Step Guide
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
What is a Scoping Review?
A scoping review is a type of research synthesis that maps the existing literature on a broad topic to identify key concepts, research gaps, and types of evidence.
This mapping exercise involves systematically searching for, identifying, and charting relevant literature to understand its characteristics, such as the volume of research, types of studies conducted, key concepts addressed, and prevalent research gaps.
Unlike systematic reviews, which aim to answer specific questions, scoping reviews are exploratory and often used to assess the extent of available evidence and inform future research directions. They involve comprehensive searches and data extraction but do not typically include a detailed synthesis of findings or a critical appraisal of study quality.
When a scoping review methodology would be appropriate:
Scoping reviews can be used as a preliminary step to a systematic review , helping to identify the types of evidence available, potential research questions, and relevant inclusion criteria.
They can save time and resources by identifying potential challenges or limitations before embarking on a full systematic review.
Scoping reviews can help clarify key concepts/definitions in the literature. If a research area has inconsistent terminology or definitions, a scoping review can map out how different concepts are used and potentially propose a unified understanding. This can help refine the focus and scope of a subsequent systematic review.
- To determine if a systematic review is feasible and worthwhile . By identifying the breadth of evidence, researchers can gauge whether there is sufficient literature to warrant a full systematic review.
- To identify gaps in the existing research . Scoping reviews can highlight areas where little or no research has been conducted, helping inform future research priorities.
- To clarify key concepts and definitions in the field . This can help refine the focus and scope of a subsequent systematic review.
- To examine how research is conducted on a certain topic . This can inform the methodology of a future systematic review
- To refine and narrow down research questions . The broad overview provided by a scoping review can help researchers develop more specific, focused questions for a systematic review.
When not to choose a scoping review methodology:
- If a systematic review already exists on the topic: A systematic review will offer a more rigorous and comprehensive analysis of the evidence if one is already available.
- Examining the range of interventions for a health condition
- Identifying types of studies conducted
- Noting populations studied
- Summarizing outcomes measured
Scoping reviews help identify areas needing further research, whereas systematic reviews aim to draw conclusions about intervention effectiveness.
Methodological Guidelines
Methodological guidelines aim to improve the consistency and transparency of scoping reviews, enabling researchers to synthesize evidence effectively.
Methodological guidelines for scoping reviews have evolved over time:
- Arksey and O’Malley (2005) proposed the initial framework.
- Levac et al. (2010) refined and extended this framework, offering more detailed guidance.
- The Joanna Briggs Institute ( JBI ) further developed the methodology, introducing a more structured and transparent process.
| Arksey and O’Malley (2005) | Levac et al. (2010) | Joanna Briggs Institute |
|---|---|---|
| 6 stages, including optional consultation; most flexible approach | 6 stages with more detailed guidance; moderate flexibility | More prescriptive approach with additional elements; most structured |
| Broad research question | Clearly articulated research question | Clearly defined research question with concept, population, and context |
| Study selection process not specified | Recommends two reviewers for study selection | Provides detailed guidance on study selection process |
| Basic data charting | More comprehensive data extraction | Detailed guidance on data extraction with specific tools |
| Basic summary of findings | Numeric summary and qualitative thematic analysis | Introduces evidence mapping for analysis |
| Quality assessment not included | Quality assessment not emphasized | Introduces potential for quality appraisal |
| Optional stakeholder consultation | Recommended stakeholder consultation | Stakeholder consultation as an integral part of the process |
| Provides basic framework | Offers enhanced detail on methodology | Provides most detailed guidance on conducting scoping reviews |
1. Developing review objective(s) & question(s)
A well-defined objective and a set of aligned research questions are crucial for a scoping review’s coherence and direction.
They guide the subsequent steps of the review process, including determining the inclusion and exclusion criteria, developing a search strategy, and guiding data extraction and analysis.
This stage involves a thoughtful and iterative process to ensure that the review’s aims and questions are explicitly stated and closely intertwined.
Defining Objectives:
This step outlines the overarching goals of the scoping review. It explains the rationale behind conducting the review and what the reviewers aim to achieve.
The objective statement should succinctly capture the essence of the review and provide a clear understanding of its purpose.
For instance, a scoping review’s objective might be to map the existing literature on a particular topic and identify knowledge gaps.
“Parents, in particular, greatly influence participation at school, at home and in the community. They undertake many actions to improve their children’s participation in daily life. Understanding the actions of parents and also their challenges and needs will contribute to how society can support these parents and thereby enable the participation of children with physical disabilities. Pediatric rehabilitation, aiming for optimal participation, could benefit from this understanding to improve Family-centered services (FCS)… However, it is unclear what kind of information is available in literature about what parents live through, do, and what kind of problems and needs they have in supporting their child’s participation? For these reasons, a scoping review was conducted in order to systematically map the research done in this area, as well as to identify any existing gaps in knowledge”
Piškur, B., Beurskens, A. J., Jongmans, M. J., Ketelaar, M., Norton, M., Frings, C. A., … & Smeets, R. J. (2012). Parents’ actions, challenges, and needs while enabling participation of children with a physical disability: a scoping review. BMC pediatrics , 12 , 1-13.
Developing Research Questions:
The research question(s) stem from the objectives and provide a focused roadmap for the review. These questions should be answerable through the scoping review process. The research question(s) should be clear, concise, and directly relevant to the overall objectives.
Using Frameworks: While not mandatory, frameworks can be helpful tools to guide the development of objectives and research questions. Frameworks like PCC (Population, Concept, Context).
- Population: Clearly define the specific group of individuals or entities that the scoping review will focus on. This could be patients, healthcare professionals, or even organizations.
- Concept: Articulate the central idea, topic, or phenomenon that the review aims to investigate. This might include interventions, diagnostic tests, or theoretical models.
- Context: Specify the setting, environment, or circumstances relevant to the research question. This could involve geographical locations, healthcare systems, or cultural contexts.
How do cultural beliefs and practices ( C -context) influence the ways in which parents ( P -parents of children with physical disabilities) perceive and address ( C -concept) their children’s physical disabilities?
What are the barriers and facilitators ( C -concept) to mental health service utilization ( C -concept) among veterans ( P -population) experiencing homelessness ( C -context)?
This scoping review aims to summarize what is known in the African scientific literature ( C -context) among cisgender persons ( P ) about a) individual experiences of GBS within health care settings ( C -concept) and b) associations between GBS experiences and health care-related outcomes ( C -concept).
What are the main theoretical and methodological characteristics ( C -concept) of the current literature ( C -context) in the area of stigma and hearing loss and stigma and hearing aids in the elderly population ( P -older adults with acquired hearing impairment), and how should future research proceed in expanding this important field of enquiry?
2. Write A Research Protocol
A research protocol is a detailed plan that outlines the methodology to be employed throughout the review process, detailing steps like documenting results, outlining search strategy, and stating the review’s objective
The protocol should be created a priori (before starting the review) to ensure transparency and reproducibility.
While not mandatory, registering your protocol is highly recommended, e.g. FigShare and Open Science Framework (OSF).
Some journals, such as the Journal of Advanced Nursing , Systematic Reviews , BMC Medical Research Methodology , BMJ Open , and JBI Evidence Synthesis , accept scoping review protocols for publication.
It’s important to note that PROSPERO, the international prospective register of systematic reviews, does not currently accept scoping review protocols for registration.
Registering a scoping review protocol is highly recommended, even if not mandatory, as it promotes transparency, reduces duplication of effort, and helps to prevent publication bias
Example Protocols:
- The nutritional care of people living with dementia at home: a protocol for a scoping study
- End-of-life care in long-term care homes: A scoping review protocol
- Delaying knee flexion following knee arthroplasty surgery: A Scoping Review Protocol
Report in the Methods Section
“Our protocol was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMAP…), which was revised by the research team and members of Health Canada, and was disseminated through our programme’s Twitter account (@KT-Canada) and newsletter to solicit additional feedback. The final protocol was registered prospectively with the Open Science Framework on 6 September 2016 ( https://osf.io/kv9hu/ ).”
Tricco, A. C., Zarin, W., Lillie, E., Pham, B., & Straus, S. E. (2017). Utility of social media and crowd-sourced data for pharmacovigilance: a scoping review protocol. BMJ open , 7 (1), e013474.
“ Our protocol was developed using the scoping review methodological framework proposed by Arksey and O’Malley (2005) [1] and further refined by the Joanna Briggs Institute [3]. The draft protocol was revised upon receiving feedback from the research team, including methodologists and healthcare providers, as well as the peer-review panel of the Canadian Institutes of Health Research. The final version of the protocol is available upon request from the corresponding author. ”
Tricco, A. C., Lillie, E., Zarin, W., O’brien, K., Colquhoun, H., Kastner, M., … & Straus, S. E. (2016). A scoping review on the conduct and reporting of scoping reviews. BMC medical research methodology , 16 , 1-10.
3. Developing eligibility criteria
This step involves developing and aligning the inclusion criteria with the objective(s) and question(s).
By providing transparent and well-justified eligibility criteria, researchers can ensure the replicability of their scoping review and allow readers to assess the relevance and appropriateness of the included sources.
When reporting eligibility criteria, emphasize the importance of clarity, justification, and a clear link to the review’s objectives.
- Describe the eligibility criteria with a rationale for why they were selected : It’s crucial to clearly articulate the specific characteristics of sources that make them eligible for inclusion in the review. Each criterion should be accompanied by a rationale explaining why it was chosen. This rationale should be grounded in scientific arguments and clearly demonstrate how the criterion aligns with the review’s objectives.
- Identify specific restrictions and provide a rationale : Restrictions, such as date range, language, or publication status, also need clear justification. For instance, limiting the review to articles published within the past ten years might be necessary to capture the most current evidence. Similarly, restricting the review to sources in a specific language, like English, should be justified, acknowledging the potential exclusion of relevant research in other languages.
When specifying the inclusion and exclusion criteria, consider the following aspects:
By using the PCC framework, researchers can systematically establish boundaries for their scoping review, ensuring that the included sources are relevant to the research question. The framework helps to ensure that the eligibility criteria are comprehensive and well-defined, enabling a more focused and meaningful synthesis of the literature
- Population : The specific characteristics of the individuals or groups being studied. For instance, a scoping review about interventions for heart failure should specify the intended patient population (e.g., adults with heart failure, elderly patients with heart failure).
- Concept : This refers to the central idea, topic, or phenomenon under investigation. In the heart failure example, the concept could be “interventions for heart failure” itself, or it could be narrowed down to a specific type of intervention, such as “exercise interventions for heart failure.”
- Context : This element considers the setting or environment in which the concept is being explored. For instance, the context of the heart failure review could be “hospital settings,” “community-based care,” or “telehealth interventions.”
It is important to note that the absence of an explicitly stated framework (e.g. PCC) does not necessarily mean that the authors did not utilize a systematic approach when developing their eligibility criteria. It is possible that they employed a framework implicitly or that their criteria development was guided by other factors.
Iterative Process
The initial set of eligibility criteria outlined in the protocol may be subject to adjustments based on the type and volume of studies identified in the initial searches.
- Initial Development : Establish preliminary inclusion and exclusion criteria at the onset of the review based on their existing knowledge of the subject area. This can be adjusted as you become more familiar with the literature and data retrieved during the search process.
- Iterative Refinement : Inclusion criteria are refined iteratively based on pilot searches and the evolving understanding of the data. This initial search is crucial as it exposes researchers to a broader range of literature, revealing additional keywords, relevant concepts, and potentially useful search terms that might not have been initially considered.
“ Studies that identified the key terms in the title, abstract, article, or MeSH heading were retained for further examination. Studies published as abstracts, conference proceedings or pilot results published in non-peer-reviewed journals were excluded. In addition, books, book chapters, comments on publications, and dissertations were also excluded. No exclusion criteria were established regarding the type of research design. Inclusion criteria were (a) older adults with progressive hearing loss being the population of interest and (b) the outcome measure was clearly focused on (or at least on some aspects of) stigma regarding hearing loss and/or hearing aids. Although given the descriptive aim of the review, no definitions of stigma and/or hearing aids were set a priori, and all articles including these terms were retrieved, the analysis of the data relied on the most common dimensions of the concept of stigma cited in the literarture: the cognitive dimension (i.e., stereotypes), the emotional dimension (i.e., prejudice) and the behavioral dime. ”
- David, D., & Werner, P. (2016). Stigma regarding hearing loss and hearing aids: A scoping review. Stigma and Health , 1 (2), 59.
“ An extensive search was conducted to locate peer-reviewed articles that addressed questions related to parent involvement in organized youth sport. To guide article retrieval, two inclusion criteria were used. First, articles were required to highlight some form of parent involvement in organized youth sport. In the present study, organized youth sport was operationalized as “adultorganized and controlled athletic programs for young people,” wherein “participants are formally organized [and] attend practices and scheduled competitions under the supervision of an adult leader” (Smoll & Smith, 2002, p. xi). In line with this criterion, we did not include physical activity, exercise, physical education, and free play settings, which comprise a substantial volume of research in sport and exercise psychology. We also excluded research that simply collected data on parents or from parents but did not explicitly assess their involvement in their children’s sport participation. Second, articles were required to have been published in peer-reviewed, Englishlanguage, academic journals. As such, we did not include books, chapters, reviews, conceptual papers, conference proceedings, theses and (Jones, 2004) dissertations, or organizational “white papers” in this scoping review. ”
Dorsch, T. E., Wright, E., Eckardt, V. C., Elliott, S., Thrower, S. N., & Knight, C. J. (2021). A history of parent involvement in organized youth sport: A scoping review. Sport, Exercise, and performance psychology , 10 (4), 536.
“…to be included in the review, papers needed to measure or focus on specific dimensions of treatment burden, developed in the conceptual framework (e.g. financial, medication, administrative, lifestyle, healthcare and time/travel). Peer-reviewed journal papers were included if they were: published between the period of 2000–2016, written in English, involved human participants and described a measure for burden of treatment, e.g. including single measurements, measuring and/or incorporating one or two dimensions of burden of treatment. Quantitative, qualitative and mixed-method studies were included in order to consider different aspects of measuring treatment burden. Papers were excluded if they did not fit into the conceptual framework of the study, focused on a communicable chronic condition, for example human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS) or substance abuse. Papers talking about carer burden, in addition to patient burden of treatment, were also included.”
Sav, A., Salehi, A., Mair, F. S., & McMillan, S. S. (2017). Measuring the burden of treatment for chronic disease: implications of a scoping review of the literature. BMC medical research methodology , 17 , 1-14.
4. Information Sources
Scoping reviews aim to identify a broad range of relevant studies, including both published and unpublished literature, to provide a comprehensive overview of the topic.
The goal is to be inclusive rather than exhaustive, which differentiates scoping reviews from systematic reviews that seek to collate all empirical evidence fitting pre-specified criteria to answer specific research questions.
Information sources for scoping reviews can include a wide range of resources like scholarly databases, unpublished literature, conference papers, books, and even expert consultations.
Report who developed and executed the search strategy, such as an information specialist or librarian. Mention if the search strategy was peer-reviewed using the Peer Review of Electronic Search Strategies (PRESS) checklist.
- Electronic Databases : Make a comprehensive list of all electronic databases you used. Common databases for health-related scoping reviews include: CINAHL, Medline, Embase, PsycINFO, SocINDEX with Full Text, and Web of Science: Core Collections.
- Specify date ranges : For each database, note the date range of your search. For example: “MEDLINE was searched from inception to July 30, 2024.”
- Grey Literature : In addition to databases, forensic or ‘expansive’ searches can be conducted. This includes: grey literature database searches (e.g. OpenGrey , WorldCat , Ethos ), conference proceedings, unpublished reports, theses , clinical trial databases , searches by names of authors of relevant publications.
- Citation chasing : If you manually searched specific journals or reference lists, document this. For example: “We hand-searched the reference lists of all included studies and relevant systematic reviews.”
- Contacting Experts : If you contacted experts in the field for additional sources, mention this: “We contacted five experts in the field of [topic] to identify any additional relevant studies.”
“To identify potentially relevant documents, the following bibliographic databases were searched from 2004 to June 2015: MEDLINE, EMBASE, LexisNexis Academic, the Legal Scholarship Network, Justis, LegalTrac, QuickLaw, and HeinOnline. The search strategies were drafted by an experienced librarian [name] and further refined through team discussion. The final search strategy for MEDLINE can be found in Additional file 3. The final search results were exported into EndNote, and duplicates were removed by a library technician. The electronic database search was supplemented by searching the Canadian Medical Protective Association website (https://www.cmpa-acpm.ca/en) and scanning relevant reviews.”
Cardoso, R., Zarin, W., Nincic, V., Barber, S. L., Gulmezoglu, A. M., Wilson, C., … & Tricco, A. C. (2017). Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Systematic reviews , 6 , 1-11.
5. Searching for the evidence
Scoping reviews typically start with a broader, more inclusive search strategy. The initial search is intentionally wide-ranging to capture the breadth of available literature on the topic
To balance breadth and depth in your initial search strategy for a scoping review, consider the following tips based on the gathered search results:
- Start with a broad initial search : Begin with a broad search across at least two relevant databases (e.g., MEDLINE and Scopus) to capture a wide range of literature. This helps identify the scope of available studies and key themes in the field .
- Test and refine your search strategy : After initial searches, review the titles and abstracts of retrieved articles to assess relevance. Analyze the text words and index terms used in these articles to refine your understanding of the topic and identify additional keywords, synonyms, and subject headings to include in subsequent searches .
- Multiple Databases : Search across a variety of databases to ensure a comprehensive literature capture. Each database may index different journals and articles, which can help broaden your search results .
- Boolean operators: The use of Boolean operators (AND/OR/NEAR/NOT) helps to combine these terms effectively, ensuring that the search strategy is both sensitive and specific. For instance, using “AND” narrows the search to include only results containing both terms, while “OR” expands it to include results containing either term.
- Truncation symbols : These broaden the search by capturing variations of a keyword. They function by locating every word that begins with a specific root. For example, if a user was researching interventions for smoking, they might use a truncation symbol to search for “smok*” to retrieve records with the words “smoke,” “smoker,” “smoking,” or “smokes.” This can save time and effort by eliminating the need to input every variation of a word into a database.
- Citation chasing : Document the specific studies whose reference lists were examined. Include the titles, authors, and publication years of these studies. Note how you identified articles that cite the studies. This could be through citation databases like Google Scholar, Scopus, or Web of Science.
- Detailed documentation : Keep thorough records of your search strategies, including the databases searched, keywords used, and any filters applied. This documentation is crucial for transparency and reproducibility .
” The planned literature search was developed on June 23, 2022. The inclusion and exclusion criteria were further refined, along with electronic databases to identify psychological and education literature (e.g., ProQuest), programs for storing data (i.e., Covidence, n.d. accessed via https://www.covidence.org/) and key search terms (e.g., resistance and transgender). The key search terms were “transgender/trans/LGBT/gender diverse/gender expansive/nonbinary,” “resistance,” and “faith/economic status/ethnicity/gender.” Daniel Abela used terms such as nonbinary, gender diverse, LGBT, and gender expansive to capture the broad spectrum of language employed in the literature when relating to individuals whose gender identification extends beyond conventional norms associated with their assigned sex at birth. Moreover, the authors wanted a diverse sample through an intersectionality lens; therefore, terms such as faith, economic status, and ethnicity were used. These terms were selected as they were deemed by all authors to be most appropriate to evaluate this study’s research question. A complete list of the final search terms and the entire electronic search strategy for the Ovid database are presented in Table 1. ”
Abela, D., Patlamazoglou, L., & Lea, S. (2024). The resistance of transgender and gender expansive people: A scoping review. Psychology of Sexual Orientation and Gender Diversity .
Ovid Search Strategy (Table 1)
- transgender.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- trans.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- LGBT.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- gender diverse.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- gender expansive.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- non-binary.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- 1 or 2 or 3 or 4 or 5 or 6
- resistance.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- faith.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- economic status.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- ethnicity.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- gender identification.mp. [mp = title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh word]
- 10 or 11 or 12 or 13
- limit 15 to (peer-reviewed journal and English language and “0110 peer-reviewed journal” and English and yr = “2012-Current”)
Search strategy can also be reported in the appendix. For example: Supplementary A: Search strategy for scoping review .
Citation Chasing Process
Citation chasing involves reviewing the reference lists of included studies and examining articles that cite those studies to identify additional relevant literature. This process helps ensure that you capture a comprehensive view of the research landscape.
If citation chasing leads to the identification of new keywords or concepts, document these adjustments and how they were incorporated into the overall search strategy.
- Document the rationale : Clearly state why citation chasing is being conducted. This could include the goal of identifying additional studies that may not have been captured through database searches or to explore the context and impact of key studies.
- Reference list review : Document the specific studies whose reference lists were examined. Include the titles, authors, and publication years of these studies.
- Citing articles : Note how you identified articles that cite the studies. This could be through citation databases like Google Scholar, Scopus, or Web of Science.
- Record number of additional studies identified : Keep a count of how many additional studies were found through citation chasing.
- A flowchart : Adapt the PRISMA flow diagram to illustrate the stages of citation chasing, the number of sources identified at each stage, and reasons for exclusion.
- Tables : Summarize key information about the sources identified through citation chasing, such as author, year, title, and reasons for inclusion or exclusion.
6. Selecting the evidence
While articles included in a scoping review are selected systematically, it is important to acknowledge that there is no assumption that the evidence reviewed is exhaustive. This is often due to limitations in the search strategy or difficulty locating specific types of sources.
The search results are screened against pre-defined eligibility criteria to determine inclusion in the review.
The goal is to identify relevant studies, with less emphasis on methodological quality. Scoping reviews generally do not appraise the quality of included studies.
Instead, scoping reviews prioritize mapping the existing literature and identifying gaps in research, regardless of the quality of the individual studies.
Two reviewers should independently screen titles and abstracts, removing duplicates and irrelevant studies based on predefined inclusion and exclusion criteria.
- Initial screening of titles and abstracts: After applying a strategy to search the literature, the next step involves screening the titles and abstracts of the identified articles against the predefined inclusion and exclusion criteria. During this initial screening, reviewers aim to identify potentially relevant studies while excluding those clearly outside the scope of the review. It is crucial to prioritize over-inclusion at this stage, meaning that reviewers should err on the side of keeping studies even if there is uncertainty about their relevance. This cautious approach helps minimize the risk of inadvertently excluding potentially valuable studies.
- Retrieving and assessing full texts: For studies which a definitive decision cannot be made based on the title and abstract alone, reviewers need to obtain the full text of the articles for a comprehensive assessment against the predefined inclusion and exclusion criteria. This stage involves meticulously reviewing the full text of each potentially relevant study to determine its eligibility definitively.
- Resolution of disagreements : In cases of disagreement between reviewers regarding a study’s eligibility, a predefined strategy involving consensus-building discussions or arbitration by a third reviewer should be in place to reach a final decision. This collaborative approach ensures a fair and impartial selection process, further strengthening the review’s reliability.
“To increase consistency among reviewers, all reviewers screened the same 50 publications, discussed the results and amended the screening and data extraction manual before beginning screening for this review. Nine reviewers working in pairs sequentially evaluated the titles, abstracts and then full text of all publications identified by our searches for potentially relevant publications. . . . We resolved disagreements on study selection and data extraction by consensus and discussion with other reviewers if needed.”
Duffett, M., Choong, K., Hartling, L., Menon, K., Thabane, L., & Cook, D. J. (2013). Randomized controlled trials in pediatric critical care: a scoping review. Critical care , 17 , 1-9.
7. Extracting the evidence
Charting, also known as data extraction, is a crucial stage in conducting a scoping review.
This process involves systematically collecting relevant information from the sources included in the review using a structured form. It is considered best practice to have at least two reviewers independently extract data from each source
Data charting in scoping reviews differs from data extraction in systematic reviews. While systematic reviews aim to synthesize the results and assess the quality of individual studies, scoping reviews focus on mapping the existing literature and identifying key concepts, themes, and gaps in the research.
Therefore, the data charting process in scoping reviews is typically broader in scope and may involve collecting a wider range of data items compared to the more focused data extraction process used in systematic reviews.
This process goes beyond simply extracting data; it involves characterizing and summarizing research evidence, which ultimately helps identify research gaps.
- Develop a Standardized Form: Creating a structured form helps to standardize the selection of sources. The form should incorporate clear questions that align with the eligibility criteria defined in the review protocol. The specific software used to create and manage the form should be specified in the review, with options such as Covidence , EndNote , or JBI SUMARI .
- Year of publication
- Origin/country of origin (where the study was published or conducted)
- Aims/purpose
- Study population and sample size (if applicable)
- Methodology/methods
- Outcomes and details of these (e.g. how measures) (if applicable)
- Key findings that relate to the scoping review question/s.
- Testing the Form: All reviewers involved in the selection process should participate in testing the standardized form. Screen the titles and abstracts of the identified articles against the predefined inclusion and exclusion criteria.
- Sample Size: A random sample of 5–10 citations can be used for the initial calibration of title and abstract screening.
- Resolving Inconsistencies: After independent screening, discrepancies between reviewers are identified and discussed. A roundtable discussion involving the review team is an effective method to address these inconsistencies and clarify any ambiguities in the form or eligibility criteria.
- Form Refinement: Based on the calibration exercise, the standardized form and its accompanying explanation should be revised and refined as needed to enhance clarity and consistency. A second calibration exercise might be necessary if the desired agreement level, typically 70%–80%, is not achieved or if reviewers require further training.
- Number of Reviewers: A minimum of two independent reviewers should be engaged in the screening process.
- Duplicate Screening: The review process should clearly state how duplicates were managed, ideally removing them before proceeding to the screening stage.
- Verification: The sources describe different approaches to verification, including independent screening by two reviewers followed by comparison of their results or a single reviewer screening followed by verification from another reviewer. The chosen approach and its rationale should be explicitly stated in the scoping review.
- Resolving Disagreements: Any disagreements arising during the screening process should be documented and resolved, ideally through discussion and consensus among the reviewers. If consensus cannot be reached, involving a third reviewer to provide an independent assessment can help in making the final decision.
- The number of reviewers involved at each stage
- How duplicates were addressed
- The software used to manage the screening process
- How disagreements were resolved
- The number of sources excluded at each stage, along with a clear rationale for their exclusion
“Search results for all databases were merged. Duplicates and nonrelated papers were excluded. Titles and abstracts of the remaining papers were assessed against the inclusion and exclusion criteria independently by both authors. The resulting papers were pooled and disagreements were resolved through discussion based on the full text article. Following this stage, a standardized form was used to summarize the information in each article. The variables extracted were: reference/ country, aim of the study, study design, year of publication, and main finding/results.”
“A data-charting form was jointly developed by two reviewers to determine which variables to extract. The two reviewers independently charted the data, discussed the results and continuously updated the data-charting form in an iterative process.”
Lenzen, S. A., Daniëls, R., van Bokhoven, M. A., van der Weijden, T., & Beurskens, A. (2017). Disentangling self-management goal setting and action planning: A scoping review. PloS one , 12 (11), e0188822.
If an article was eligible for inclusion in this study, data related to the patient-centered care framework or model presented in the article was extracted by the lead author and reviewed by a second author (JCM). Data extracted from the reviewed patient-centered care frameworks and models was entered into data extraction records and synthesized in summary format. Data were systematically charted using the data charting form developed in Microsoft Excel. Information on authorship, article type, population, and patientcentered care approach were recorded on this form. A second data charting form was developed to chart data on the communication systematic reviews identified. Information on clinical context, patient-centered care focus, number of studies reviewed and key findings were recorded on this form.
Constand, M. K., MacDermid, J. C., Dal Bello-Haas, V., & Law, M. (2014). Scoping review of patient-centered care approaches in healthcare. BMC health services research , 14 , 1-9.
The final charting form, which clearly defines each item, should be included in the scoping review as an appendix or supplementary file, if possible.
- Author: This information is essential for referencing and should be consistent throughout the scoping review document.
- Year of Publication: Noting the publication year of each source helps analyze trends and changes in research over time. This variable can highlight areas where research has progressed or where further investigation is necessary.
- Country: This variable involves noting the country of the study and the bibliographic details of each source. The country of origin provides context and helps assess the generalizability of findings to other settings.
- Objective(s): The objectives of each included source of evidence should be clearly stated. This variable helps understand the aim of each study and how it contributes to the overall scoping review question.
- Participants (characteristics/total number): This variable involves describing the defining characteristics of the participants in the included sources of evidence. Details like diagnostic criteria, age, ethnicity, and the total number of participants are crucial elements of this variable. This information provides context to the scoping review findings.
- Concept: This variable pertains to extracting and mapping data related to the core concept being investigated in the scoping review. The specific data extracted will depend on the nature of the concept, which should be clearly defined in the scoping review.
- Intervention Type: If applicable to the scoping review question, the type of intervention used in each source should be recorded. This might include details like the specific intervention method, the comparator used, and the duration of the intervention. This information helps compare and contrast different interventions explored in the included studies.
- Methodology: Describing the methodology employed by each source is essential to understand how the research was conducted. This variable provides insights into the study design, data collection methods, and analysis techniques used. Categorizing study designs is essential to compare and contrast different research approaches and their potential implications for the scoping review’s conclusions.
- Outcome Measures: This variable focuses on the tools or methods used to assess the effects of an intervention or phenomenon. It’s essential to describe the specific outcome measures used in each study, including details on how they were measured. This information helps compare findings across studies using similar outcome assessment tools.
- Main Finding: This variable focuses on extracting the primary findings or results of each study that are relevant to the scoping review’s research question. These findings form the core evidence base and are crucial for addressing the scoping review objectives.
“We abstracted data on article characteristics (e.g., country of origin, funder), engagement characteristics and contextual factors (e.g., type of knowledge user, country income level, type of engagement activity, frequency and intensity of engagement, use of a framework to inform the intervention), barriers and facilitators to engagement, and results of any formal assessment of engagement (e.g., attitudes, beliefs, knowledge, benefits, unintended consequences).”
Tricco, A. C., Zarin, W., Rios, P., Nincic, V., Khan, P. A., Ghassemi, M., … & Langlois, E. V. (2018). Engaging policy-makers, health system managers, and policy analysts in the knowledge synthesis process: a scoping review. Implementation Science , 13 , 1-19.
8. Analyzing the evidence
The key element of a scoping review is the synthesis: that is the process that brings together the findings from the set of included studies in order to draw conclusions based on the body of evidence.
Data synthesis in a scoping review involves collating, combining, and summarizing findings from the included studies.
This process aims to provide a reliable and comprehensive answer to the review question by considering the strength of the evidence, examining the consistency of observed effects, and investigating any inconsistencies.
The data synthesis will be presented in the results section of the scoping review.
- Develop a clear text narrative that explains the key findings
- Use a logical heading structure to guide readers through your results synthesis
- Use tables to summarise findings (can be same table as data extraction)
Scoping reviews often use a more descriptive approach to synthesis, summarizing the types of evidence available, key findings, and research gaps.
- Research design (e.g., experimental, observational, qualitative)
- Population characteristics
- Intervention types
- Outcome measures
- Theoretical frameworks
- Geographic regions
- Time periods
- The predominant study designs used in the field
- The range of methodologies employed
- The diversity (or lack thereof) in research approaches
- Primary outcomes
- Major conclusions drawn by the authors
- Any notable or unexpected findings
- Recurring themes in the literature
- Evolving research focuses over time
- Commonly used methodologies or theoretical frameworks
- Consistency (or inconsistency) in findings across different studies
- Identifying areas that have been extensively studied
- Noting topics that have received less attention
- Highlighting any shifts in research focus over time
- Populations that have been understudied
- Methodologies that haven’t been widely applied
- Questions that remain unanswered or inadequately addressed
- Contradictions in the literature that need further investigation
- Summarizing key concepts: Identify and describe the central ideas, theories, or constructs that emerge from the literature. This helps to provide a conceptual overview of the field.
- Tables summarizing study characteristics
- Charts showing the distribution of studies across categories
- Concept maps illustrating relationships between key ideas
Remember, the goal in a scoping review is not to critically appraise the quality of individual studies or to provide a definitive answer to a narrow research question.
Instead, the synthesis aims to provide a broad overview of the field, mapping out the existing literature and identifying areas for further research.
This descriptive approach allows for a comprehensive understanding of the landscape of a particular research area.
“We grouped the studies by the types of behavior they analyzed, and summarized the type of settings, populations and study designs for each group, along with the measures used and broad findings. Where we identified a systematic review, we counted the number of studies included in the review that potentially met our inclusion criteria and noted how many studies had been missed by our search.”
Hutchinson, J., Prady, S. L., Smith, M. A., White, P. C., & Graham, H. M. (2015). A scoping review of observational studies examining relationships between environmental behaviors and health behaviors. International journal of environmental research and public health , 12 (5), 4833-4858.
9. Presenting the results
The findings should be presented in a clear and logical way that answers the research question(s). This section might include tables, figures, or narrative summaries to illustrate the data.
Narrative Summaries
Write a clear, concise narrative that brings together all of these elements. This should provide readers with a comprehensive overview of the current state of knowledge in the field, highlighting both what is known and what remains to be explored.
The primary goal of a narrative summary is to weave together the information extracted from multiple sources into a cohesive and understandable narrative. This story should focus on why a specific action is necessary, should be discontinued, or lacks sufficient evidence to determine its efficacy
A well-crafted narrative summary often utilizes headings and subheadings to organize the synthesized information logically.
This approach makes it easier for readers to follow the thought process and understand the relationships between different pieces of evidence.
Strategies on how to be sensitive to patient needs were primarily discussed in the qualitative research articles included in this review. Such strategies included acknowledging and adapting to unique patient identifiers [19,24,25]. For example, clinicians are urged to observe and reflect on fluctuating levels of patient alertness, patient comfort levels in the presence or absence of family members, and different communication barriers such as hearing loss, in order to facilitate clinical interactions [15,19,22]. Of the articles reviewed, 58% identified that careful observation of unique patient characteristics is necessary to providing care that will lead to optimal patient receptiveness and positive health outcomes.
While narrative summaries primarily use text, incorporating tables, charts, or diagrams can enhance clarity, particularly when presenting complex data patterns.
However, always accompany these visual aids with a clear textual explanation to ensure comprehensive understanding.

PRISMA Flowchart
Using a PRISMA flowchart in a scoping review is considered good practice. It promotes transparency and allows for a clear understanding of how sources were selected.
The flowchart illustrates the step-by-step process of screening, filtering, and selecting studies based on predefined inclusion and exclusion criteria.
The flowchart visually depicts the following stages:
- Identification: The initial number of titles and abstracts identified through database searches.
- Screening: The screening process, based on titles and abstracts.
- Eligibility: Full-text copies of the remaining records are retrieved and assessed for eligibility.
- Inclusion: Applying the predefined inclusion criteria resulted in the inclusion of publications that met all the criteria for the review.
- Exclusion: The flowchart details the reasons for excluding the remaining records.
Petersen, B., Koshy-Chenthittayil, S., DeArmond, M., & Caromile, L. A. (2023). Assessment of diversity-based approaches used by American Universities to increase recruitment and retention of biomedical sciences research faculty members: A scoping review protocol. Plos one , 18 (6), e0276089.
10. Discussion Section And Conclusion
Summarizing the evidence in relation to the purpose of the review, making conclusions and noting any implications of the findings.
It is also essential to remember that scoping reviews, unlike systematic reviews, do not aim to provide concrete recommendations for practice or policy.
Their primary function is to map the existing evidence, identify knowledge gaps, and clarify concepts, rather than synthesize results for direct application in clinical or policy settings
Summarizing the Evidence
- Summarize key findings in relation to your research questions
- Highlight main themes or patterns across studies
- Explain the nuances and complexities in the evidence
- Tailor overall findings of the scoping review to the relevant knowledge users such as policymakers, health care providers and patients or consumers
- Discuss the consistency of the evidence
- This provides a clear takeaway message for readers
“In this scoping review we identified 88 primary studies addressing dissemination and implementation research across various settings of dementia care published between 1998 and 2015. Our findings indicate a paucity of research focusing specifically on dissemination of knowledge within dementia care and a limited number of studies on implementation in this area. We also found that training and educating professionals, developing stakeholder interrelationships, and using evaluative and iterative strategies are frequently employed to introduce and promote change in practice. However, although important and feasible, these strategies only partly address what is repeatedly highlighted in the evidence base: that organisational factors are reported as the main barrier to implementation of knowledge within dementia care. Moreover, included studies clearly support an increased effort to improve the quality of dementia care provided in residential settings in the last decade.”
Lourida, I., Abbott, R. A., Rogers, M., Lang, I. A., Stein, K., Kent, B., & Thompson Coon, J. (2017). Dissemination and implementation research in dementia care: a systematic scoping review and evidence map. BMC geriatrics , 17 , 1-12.
Limitations
When considering the limitations of a review process, particularly scoping reviews, it’s essential to acknowledge that the goal is breadth, not depth, of information.
This means that unlike systematic reviews, scoping reviews generally don’t involve a formal appraisal of the methodological quality of included studies, unless specifically required by the review’s aim.
- One significant limitation frequently encountered in reviews is the restriction to English-language sources. This decision, often made for feasibility, can inadvertently introduce bias by excluding valuable research from non-English speaking communities and potentially limiting the generalizability of the findings.
- For instance, if a scoping review protocol initially excludes gray literature but later incorporates it due to the emergence of relevant findings during the review process, this change needs to be explicitly stated and justified in the final report.
“Our scoping review has some limitations. To make our review more feasible, we were only able to include a random sample of rapid reviews from websites of rapid review producers. Further adding to this issue is that many rapid reviews contain proprietary information and are not publicly available. As such, our results are only likely generalizable to rapid reviews that are publicly available. Furthermore, this scoping review was an enormous undertaking and our results are only up to date as of May 2013.”
Tricco, A. C., Antony, J., Zarin, W., Strifler, L., Ghassemi, M., Ivory, J., … & Straus, S. E. (2015). A scoping review of rapid review methods. BMC medicine , 13 , 1-15.
Conclusions
Discuss implications:.
- Note that recommendations for practice and policy will not be relevant for most scoping reviews as the goal is to provide a preliminary map of the evidence without appraising the quality and validity of the results.
- Consider both positive and negative implications.
- This helps translate your findings into real-world applications.
Identify gaps and future research:
- Point out areas where evidence is lacking or inconsistent.
- Suggest specific research questions or study designs to address these gaps.
- Recommendations for future research are often a key element, particularly suggestions for more focused systematic reviews based on the scoping review’s findings.
- For instance, a scoping review might reveal a need for research linking specific features of expertise to mental and physical health outcomes. Similarly, there might be methodological gaps regarding the validation of certain measures or understanding experiences across diverse contexts and populations.
“The lack of evidence to support physiotherapy interventions for this population appears to pose a challenge to physiotherapists. The aim of this scoping review was to identify gaps in the literature which may guide a future systematic review. However, the lack of evidence found means that undertaking a systematic review is not appropriate or necessary […]. This advocates high quality research being needed to determine what physiotherapy techniques may be of benefit for this population and to help guide physiotherapists as how to deliver this.”
Hall, A. J., Lang, I. A., Endacott, R., Hall, A., & Goodwin, V. A. (2017). Physiotherapy interventions for people with dementia and a hip fracture—a scoping review of the literature. Physiotherapy , 103 (4), 361-368.
Potential Challenges
- Balancing breadth and depth: Scoping reviews necessitate a careful balance between covering a wide range of literature (breadth) and providing sufficient depth of analysis. A scope that is too broad can become unmanageable and result in superficial treatment of the topic. Conversely, excessive focus on depth might compromise the comprehensiveness of the review. This balance requires careful consideration during the planning stages, particularly when defining the review question and inclusion criteria.
- Lack of standardized terminology and methods: While frameworks for scoping reviews exist, there is still a lack of consensus on terminology and methods, potentially leading to inconsistencies in how they are conducted and reported. This variability can make it challenging to assess the quality and reliability of scoping review findings.
- Difficulty in analyzing and presenting findings: Scoping reviews often involve synthesizing information from a large and diverse body of literature. Analyzing and presenting this information in a meaningful and concise way can be demanding, requiring a high level of analytical skill and clarity of presentation. The absence of standardized analysis methods further exacerbates this challenge, leading to potential inconsistencies in how data is extracted, analyzed, and presented.
- Limited resources and time constraints: Scoping reviews, although sometimes perceived as a quicker alternative to systematic reviews, can still be resource-intensive. They require meticulous planning, comprehensive searching, and rigorous analysis.
Reading List
- Arksey, H., & O’Malley, L. (2005). Scoping studies: towards a methodological framework . International journal of social research methodology , 8 (1), 19-32.
- Levac, D., Colquhoun, H., & O’brien, K. K. (2010). Scoping studies: advancing the methodology . Implementation science , 5 , 1-9.
- Munn, Z., Peters, M. D., Stern, C., Tufanaru, C., McArthur, A., & Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology , 18 , 1-7.
- Pearson, A., Wiechula, R., & Lockwood, C. (2005). The JBI model of evidence-based healthcare. JBI Evidence Implementation , 3 (8), 207-215.
- Peters, M. D., Godfrey, C., McInerney, P., Munn, Z., Tricco, A. C., & Khalil, H. (2020). Scoping reviews . JBI manual for evidence synthesis , 10 .
- Peters, M., Godfrey, C., McInerney, P., Soares, C. B., Khalil, H., & Parker, D. (2015). Methodology for JBI scoping reviews. In The Joanna Briggs institute reviewers manual 2015 (pp. 3-24). Joanna Briggs Institute.
- Peters, M., Godfrey, C., Khalil, H., McInerney, P., Soares, C., & Parker, D. (2017). 2017 guidance for the conduct of JBI scoping reviews . Joana Briggs Inst Rev Man , 13 , 141-6.
- Pollock, D., Davies, E. L., Peters, M. D., Tricco, A. C., Alexander, L., McInerney, P., … & Munn, Z. (2021). Undertaking a scoping review: A practical guide for nursing and midwifery students, clinicians, researchers, and academics . Journal of advanced nursing , 77 (4), 2102-2113.
- Pollock, D., Peters, M. D., Khalil, H., McInerney, P., Alexander, L., Tricco, A. C., … & Munn, Z. (2023). Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI evidence synthesis , 21 (3), 520-532.
- Scott, H., Sweet, L., Strauch, L., & Muller, A. (2019). Expressed breastmilk handling and storage guidelines available to mothers in the community: A scoping review. Women and Birth, 33 (5), 426–432.
- Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, Moher, D, Peters, MD, Horsley, T, Weeks, L, Hempel, S et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018,169(7):467-473.
- Open access
- Published: 31 July 2024
Rare disease clinical trials in the European Union: navigating regulatory and clinical challenges
- Sangita Mishra ORCID: orcid.org/0000-0002-7879-5259 1 &
- MP Venkatesh ORCID: orcid.org/0000-0002-6804-1023 1 , 2
Orphanet Journal of Rare Diseases volume 19 , Article number: 285 ( 2024 ) Cite this article
338 Accesses
1 Altmetric
Metrics details
Clinical development for orphan drugs presents significant difficulties and challenges. There is no unique or standard design, conduct, and outcome assessment methodology and it is sometimes impractical to fit design models of rare disease trials in any practiced and well-known framework. In the European Union (EU) these challenges encompass a broad array of subjects, including trial design, study outcomes, patient recruitment, trial conduct ethics, trial cost, and chances of success. This literature-based review study aims to provide a thorough overview of the critical aspects of rare disease trials in the EU by analyzing the current landscape of rare disease trials, highlighting key challenges, delving into regulatory and research initiatives and innovation in trial designs, and proposing multi-faceted solutions to implement effective rare disease clinical trials in the region.
Traditional clinical trial designs, validation, and evaluation methodologies used for nonorphan drugs often prove unsuitable for orphan drugs, given the small patient populations, sometimes fewer than 1000 cases. There is an increasing need for accessible therapies and both regulators as well as industry are trying to develop affordable and effective drugs to address this need. Despite several steps that have been taken, the timely development of drugs remains a challenge. One of the reasons behind the long development timeline is the recruitment, retention, and conduct of rare disease trials. To optimize the development timelines of orphan drugs in the EU, it is important to ensure that the safety and efficacy of the product is not compromised. Industry and regulatory agencies must implement innovative trial designs, devise flexible policies, and incorporate real-world data for assessing clinical outcomes.
Collaboration among academic institutions, pharmaceutical companies (both small and major), patient groups, and health authorities is crucial in overcoming obstacles related to clinical trials and providing assistance and creative ideas. The ultimate objective of granting rare disease patients timely and affordable access to medications with a positive balance between benefits and risks is to be met.
Developing effective treatments for rare diseases is challenging due to their low prevalence in the EU, with only 5 patients per 10,000. Most rare diseases lack adequate treatment options. Conducting clinical trials with small populations is challenging resulting in limited evidence generation. To address this issue, regulatory guidance, such as the “Guideline on clinical trials in small populations” [ 1 ] by the European Medicines Agency (EMA) guidance in the EU, covers various aspects of clinical trials, including pharmacological factors, endpoint selection, control group selection, methodological considerations, statistical considerations, and levels of evidence. In 2012, the European Commission (EC) launched a call for proposals, “New methodologies for clinical trials for small population groups,” under the FP7 health innovation framework [ 2 ]. This initiative aimed to develop improved statistical methods for assessing the safety and effectiveness of treatments for small population groups, focusing on rare diseases and personalized medicine and increasing evidence generation from the clinical trials to support safety and efficacy outcomes. The objective was to reduce trial design costs and conduct effective clinical trials that produce reliable outcomes for rare disease studies involving small patient populations [ 3 ]. Randomized clinical trials have been tried on rare disease trials in the EU, but since the population of patients is small the question of appropriate sample inclusion and impact of outcomes of these trials in the long run cannot be surely concluded [ 3 ]. In recent times, innovative clinical trial designs using advanced statistical methods, simulation programs to emulate real-life trial designs and scenarios, and usage of real-world evidence (RWE) to assess and determine clinical endpoints for study design are some of the approaches proposed by regulators.
Significance of the study
This study employs a review of various literature types encompassing review papers, concept papers, points of view, empirical researchers, policy guidelines, and expert reviews to explore the challenges faced by researchers in conducting rare disease trials in the EU as well as patient perspectives, and regulatory hurdles.
It aims to provide key insights by incorporating views and findings from various sources by adopting a fresh perspective considering the evolving landscape of rare disease policies and increasing focus on orphan drug research based on the latest scientific advancements. This study also aims to introduce actionable recommendations beyond theoretical practices and general solutions by incorporating suggestions based on technical advancements in health tracking, disease progression modeling using natural history data, and treatment outcome assessments and provides perspectives for conducting future research in this domain. It focuses on the specific regional nuances of the EU and attempts to identify strategies that will fit into the orphan drug development and regulatory framework of this region.
This study adopts an interdisciplinary approach to promote innovative trial designs led by improved policy decisions, simulation modeling and effective usage of registry and natural history data, novel endpoint and biomarker-based effectiveness assessments, adopting innovative drug development planning, and improving collaboration across the healthcare value chain.
Methodology
Study selection and data collection.
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Refer PRISMA Checklist for details. The criteria for the selection of relevant studies and documents and for performing the necessary assessment of relevant literature are provided below.
Search strategy and selection criteria
Search was performed to retrieve relevant studies, policy documents, and online materials from PubMed, SCOPUS, BMJ, JAMA, Nature, Web of Science collection, Taylor and Francis Online, and internet search. Search terms were categorized into three parts:
Population: “rare disease OR orphan drugs” AND (Europe OR European Union OR EU OR “multicountry”).
Intervention: “clinical trial” OR “clinical research” OR “drug development”.
Outcomes: “regulatory challenges” OR “regulatory hurdles” OR “innovative designs” OR “patient recruitment” OR “trial design” OR “methodology” OR “regulatory strategies” OR “real-world data” OR “natural history data”.
The indexing was performed using the following search query:
(“rare disease” OR “orphan drug “) AND (“Europe” OR “European Union” OR “EU” OR “multicountry”) AND ((“clinical trial” OR “clinical research” OR “drug development”) AND (((“regulatory challenge” OR “regulatory hurdle”) OR “regulatory strategy” OR (“innovative design” OR “trial design” OR “methodology”) OR (“patient recruitment” OR “patient engagement”) OR (“real world data” OR “real world evidence”) OR “natural history data”).
The data related to the clinical trial landscape were extracted from the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization and the EU-related data were filtered based on the EU Clinical Trial registry.
We tailored data extraction to fit the study’s qualitative and narrative nature, systematically retrieving key meta-data like document details, publication dates, objectives, designs, policies, shortcomings, opportunities, and findings. Two independent reviewers ensured the accuracy of data by reviewing various aspects covered in the documents.
Inclusion and exclusion criteria
The content considered for inclusion comprised studies that report on the regulatory and clinical challenges of conducting rare disease clinical trials in the EU and focused on regulatory and innovative strategies to address the same, studies including commentaries, reviews, and editorials that provide recommendations or solutions to overcome the challenges of rare disease clinical trials in the EU, studies that are published in English or have an English abstract available and studies that are published from 2005 onwards, to reflect the evolving regulatory and clinical landscape of rare disease research and trials in the EU. Also, articles and web publications in peer-reviewed journals or websites and from reputable organizations were considered for study.
Studies that do not focus on rare disease clinical trials, or only mention them as a secondary or minor aspect, studies that did not address the EU context, or only mention it as a secondary or minor aspect, Studies that provided a generic overview of these aspects and have methodological limitations and studies not directly addressing the specifics of the challenges and not providing any insight into innovative trials and clinical evaluation or planning were excluded from the study.
23 journal articles and 30 web publications (position papers, editorials, frameworks, guidelines, and details of stakeholder organizations in the EU rare disease landscape) were considered to conduct this study.
The research focus and findings of the 23 journal articles are provided in Table 1 . Please refer below:
The studies of rare disease trials have proposed key conceptual frameworks and have also provided empirical insights into the design, conduct, evaluation, and outcome of these trials. A baseline synthesisation of key findings from these studies provides a core observation that the design and conduct of clinical trials can be assumed to be centered around three themes: Disease characteristics, Trial Design and Methodology, and Clinical Outcome Assessment. The core concept of clinical research plans begins with understanding specific rare diseases, their heterogeneity, and natural history, which inform Trial Design and Methodology, including study objectives, endpoints, and ethical considerations. Clinical outcomes and impacts, such as efficacy, safety, patient-reported outcomes, and cost-effectiveness, are influenced by the chosen methodology and may vary across different ethnic groups and even within patients at various stages of the disease or encountering specific molecular subsets of the disease. Relationships between these themes highlight the iterative nature of clinical research and especially in the case of rare diseases these iterations may not be sequential but in most cases need to be concurrent and flexible to ensure maximum and diverse patient coverage. Incorporating the themes in a common regulatory and funding framework across the EU becomes challenging due to huge ethnic, geographical, financial, infrastructural, and cultural diversity. Stakeholder perspectives, comparative analysis, ethical considerations, and future directions add depth to ongoing drug development initiatives. This approach warrants the inclusion of diverse viewpoints, ethical awareness, ongoing refinement of research practices, and continuous innovation in the design and conduct of trials to address the unique challenges and opportunities in rare disease clinical trials within the EU.
Landscape of orphan drug clinical trials in the EU:
Clinical trials conducted based on therapeutic areas in the eu.
Between 2007 and 2022, 152 clinical trials were conducted on orphan drugs in the EU, as recorded in the EU Clinical Trial Registry. Refer to Fig. 1 . 23% of the trials were on cancer-related therapies, followed by blood disorders with an 18% share of the trials and congenital abnormalities with a 14% share of the trials. The rest of the trials were related to other therapeutic areas, such as cardiovascular diseases, immune system diseases, and nervous disorders.
Orphan drug clinical trials based on therapeutic area (2007–2022) (EU Clinical Trial Registry). Source International Clinical Trials Registry Platform (ICTRP) [ 9 ]
Yearly spread of clinical trials of orphan drugs in the EU
Between 2007 and 2022, clinical trials on orphan drugs have seen both an increase and a decrease on a year-on-year basis, with a decrease observed during the intermittent period mainly in 2015 and 2016. Refer to Fig. 2 . After that, the trend has been upward again, with 22 trials being conducted in 2021, which is the highest in the period under observation. The number again decreased to 12 in 2022. There is an average increase of 51% in the clinical trial volume from 2007 to 2022.
Yearly orphan drug clinical trials in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [ 9 ]
Clinical trial volume of orphan drugs based on product types in the EU
Between 2007 and 2022, biological products underwent 78 trials compared to 71 trials for synthetic drugs. Refer to Fig. 3 . This might be an indication that investments made by the EU in the field of biotechnology have created a favorable environment for sponsors for biologic development. The EU market provided potential commercial opportunities due to less competition in the field of biologics. Biologics may also be favored due to their targeted mechanism of action, high specificity and efficacy, and ability to modulate complex biological processes underlying the specific disease being treated. These factors make biologics more suitable therapies than synthetic drugs for diseases belonging to certain therapeutic areas.
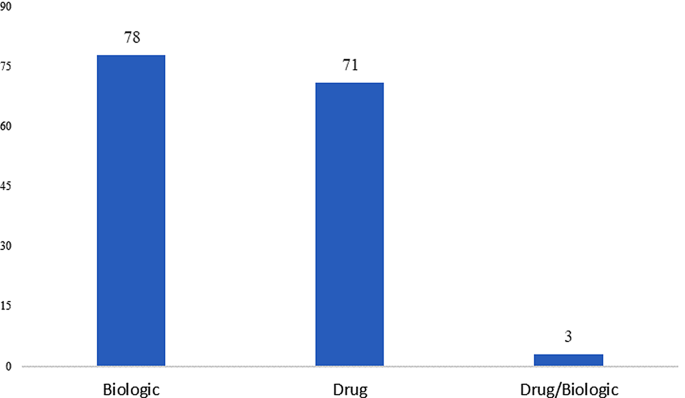
Orphan drug trial volume based on product type in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [ 9 ]
Top 10 companies conducting orphan drug clinical trials in the EU
Between 2007 and 2022, Novo Nordisk conducted the maximum number of clinical trials focusing on congenital, hereditary, and neonatal diseases and abnormalities, nutritional and metabolic diseases, digestive system diseases, cardiovascular diseases, and blood and nervous disorders. It was followed by BioCryst Pharmaceuticals Inc., which conducted 19 trials focusing on blood disorders and immune system diseases. The top 10 companies contributed to 64% of all orphan drug clinical trials in the EU between 2007 and 2022. Refer to Fig. 4 .
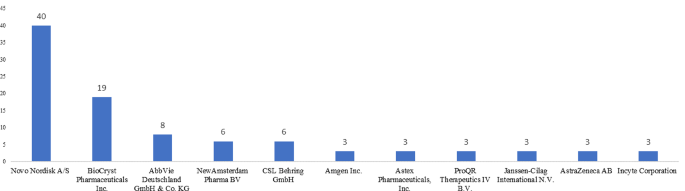
Top 10 companies in terms of orphan drug trials in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [ 9 ]
Clinical trials on biologics have seen an increase between 2019 and 2022 primarily due to an increased focus on biologics development for rare disease therapies. This is also backed by the growing knowledge of genetic markers of specific diseases that help in the development of targeted therapies for patients. It was observed that 45% of all biologic therapeutic trials between 2007 and 2022, were carried out between 2019 and 2022. Refer to Fig. 5 .
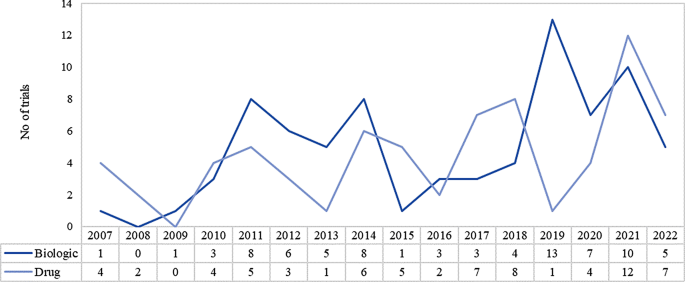
Yearly trend of clinical trials of orphan drugs based on product types in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [ 9 ]
Inferences from landscape data
Companies have been showing an increased interest in orphan drug development in the EU, primarily supported by the EMA’s orphan drug development incentives such as ten years of market exclusivity for orphan-designated products, centralized and accelerated review of marketing authorization applications for orphan products, conditional marketing authorization of certain drug types, compassionate access under exceptional circumstances for patients with high morbidity, application and regulatory fee waivers and EC research frameworks and grants for orphan drug innovation and rare disease natural history studies. Most of the major pharmaceutical companies have developed and conducted trials on a considerable number of orphan drugs across different therapeutic areas. This, supported by collaborative programs by European Reference Networks (ERNs), the European Joint Program on Rare Diseases (EJP-RD) [ 10 , 11 ], and the International Rare Diseases Research Consortium (IRDiRC) [ 12 ] has played a major role in creating a translational research environment for orphan drug development and innovative trial design. Many small and medium enterprises (SMEs) working on rare disease innovation in the biotechnology and pharmaceutical domain are supported by the European Confederation of Pharmaceutical Entrepreneurs (EUCOPE) in terms of financial incentives, development support, and scientific advisory. EUCOPE also acts as a bridge for advancing translational research. Qualified SMEs receive additional incentives from the EMA like a 100% fee reduction for administrative and procedural assistance, pre-authorization inspection, initial marketing authorization application and post-authorization applications, and annual fee, specified in Council Regulation (EC) No 297/9554, in the first year from granting of a marketing authorization. The development of unified patient databases through the European Rare Disease Registry Infrastructure (ERDRI) and Patient-Reported Outcome and Quality of Life Instruments Database (PROQOLID) [ 13 ] database by Mapi Research Trust has provided valuable information on Real World Data from patient treatment outcomes, valuable biomarkers, diagnostic reports, and observer as well patient experience feedback [ 14 ]. Although there have been definitive steps taken at the EC level, regulatory agency level, and by research consortiums and patient organizations such as the European Organization for Rare Diseases (EURORDIS) [ 15 ], the pace of innovation and marketing approval of drugs is not as expected, as is evident from the overall analysis presented in the landscape section Apart from the practical challenges that are discussed in a later section, ineffective utilization of regulatory incentives and the requirement of high capital investment have been factors behind a slower pace of therapeutic development. Along with the key requirements of addressing clinical trial issues, it is equally important to implement an alternative approach to drug development planning to increase the participation of players in orphan drug clinical research and development.
Key aspects of rare disease trial designs
Practical challenges in conducting orphan drug clinical trials in the european union.
Clinical trials, being one of the most significant stages in the drug development lifecycle face various hurdles, especially in a multi-country setup like the EU. It includes finding eligible patients and overcoming awareness issues. International trials introduce further complexities like consensus on diagnosis and cultural considerations [ 4 ] Disease heterogeneity poses further challenges making progression tracking difficult, and varied manifestations across different patient groups create challenges in diagnosis, treatment, and understanding effectiveness [ 5 ]. Genetic factors, variability in disease frequency among patient groups [ 3 ], lack of knowledge about disease progression [ 7 ], and co-existing illnesses complicate the determination of inclusion/exclusion criteria and the tenure of trials [ 8 , 16 ]. In many cases, RCTs fail to deliver the desired trial outcomes in normal settings. Limited knowledge about appropriate endpoints complicates protocol design, hindering regulatory approval. Small patient populations and data variability exacerbate challenges in demonstrating drug effectiveness and safety, compounded by the absence of standardized clinical trial templates for most rare [ 6 , 17 ]. Balancing risks and benefits, respecting autonomy, and ensuring equal access are crucial issues. The limited treatment options make patients more likely to accept risks, raising concerns about informed consent which becomes a critical ethical issue [ 17 ]. Evolving regulations with stricter requirements and limited expert resources can delay research [ 18 ]. Regulatory processes and differing policies across countries add complexity to the design and conduct of rare disease trials in the EU [ 19 ]. Regulators must rely on literature review information and empirical data to a large extent when making decisions. Data extrapolation is followed for diseases with existing data, while for diseases with little prior data, several iterations need to be performed across different patient population samples resulting in cost overruns or high expenditure for the sponsors. A considerable patient population of rare disease patients is children, posing challenges in recruitment, retention, and management due to factors like developmental, emotional, and family dynamics. The varying clinical research approaches for pediatric and adult use intensify complexities in recruitment criteria, consent, and regulatory acceptance of study outcomes, amplifying oversight of ethical aspects in conducting trials [ 20 ]. In terms of costs, rare disease clinical trials incur high costs due to challenges such as the geographical spread of patients, complex trial protocols, and expensive manufacturing overheads to meet regulatory standards. Specialized trial designs may be necessary to recruit the required number of patients, further escalating expenses. Additionally, data collection and analysis using sophisticated statistical models, drive up the overall cost of drug development. Hence, this factor becomes one of the key components in drug pricing, treatment availability, and reimbursement as well. Countries like Germany, with a strong economy and financial budget, have the privilege to establish initial prices of innovative therapies making it one of the initial markets for product launch. This results in a price that may be attractive to the sponsor company but may pose a significant financial burden on countries with less financial resources and undeveloped reimbursement schemes. The downside of high pricing needs to be considered as well. Any kind of failure in price negotiations for high-cost therapies with the national Governments results in the withdrawal of the therapeutic product from the entire EU market due to a lack of reference and attractive pricing for the company. An example of this was the withdrawal of Zynteglo, for the treatment of severe Beta thalassemia and of Skysona, for the treatment of Cerebral adrenoleukodystrophy by Bluebird Bio. Pricing remains a critical issue in rare disease drug development and treatment availability [ 21 ]. One approach to mitigate the cost issue, is to implement specialized National Action Plans for rare diseases as per recommendations of the European Council. From an economic point of view, it seems difficult to implement uniform research plans, funding mechanisms, and reimbursement schemes across all member states. To address this, the European Committee of Experts on Rare Diseases (EUCERD) came out with EUROPLAN indicators to monitor the progress of plans in individual member states. These indicators can help track the progress of the National Action Plans. Stronger economies can provide financial and administrative expertise and financial grants through the E-Rare (ERA-NET) research program on rare diseases. An increased public-private partnership should be encouraged to enhance the expertise in conducting clinical research through a multi-stakeholder collaborative approach. This will provide an initial booster to the weaker economies to streamline their action plans and frame appropriate funding and reimbursement mechanisms for rare disease drug development and treatment.
Technical aspects of clinical trial design for rare diseases
RCTs have been the globally accepted and most reliable clinical trial design among clinical researchers, investigators, and regulatory agencies for demonstrating the effectiveness of a drug. However, conducting multiple RCTs can be time-consuming, and expensive, and may not fully reflect real-world clinical settings. As a result, there is a growing interest in finding innovative approaches to enhance the efficiency of clinical research [ 22 ]. RCTs are designed with standardized and comprehensive outcome measurements to determine the safety of the drug under trial. RCTs generate substantial evidence to determine the effectiveness of the drug by incorporating bias-reduction techniques to reduce errors in observations [ 22 ].
Randomization helps to differentiate between treatment outcomes as well as the variability of outcomes within a group. However, despite the multiple benefits and robustness of the design of RCTs, the validity of these cannot be always confirmed in orphan drug scenarios due to the small patient population. This is due to sampling techniques being unable to provide sufficient observation points. The design of orphan drug trials requires multicentre coordination across different locations in the world [ 8 ]. This requires the design and analysis of innovative trials through appropriate randomization procedures for a smaller population of patients. As the patient population varies across diseases as well as across demography or geography, there are always differing amounts of bias. Hence, it is imperative that no unique procedure or randomization technique is applicable, and it should be supported by proper design methodology, statistical tests, and analytical methods [ 3 ].
Rare disease clinical trial design considerations
Randomized trials are highly dependent on patient registry information to identify specific representative populations. Periodic reference to the updated registries can help sponsors improve study design and determine study cohorts to conduct case-control and observational studies. This helps in bias reduction but there are also certain challenges in terms of rare disease trial design. Existing registries may not have adequate information and validation of the correctness becomes a challenge due to highly fragmented content, lack of expertise, and data sharing or privacy controls. From a disease perspective, the progression of rare diseases is highly heterogeneous and varies between demographics and geographical dispersions. Genetic markers within the same geographical area can vary between communities with few communities highly susceptible to the disease due to cultural or lifestyle practices. In this scenario, it is difficult to determine the proper natural history and clinical endpoint based on diagnostic and treatment outcomes [ 3 ].
Analytical and statistical considerations for clinical trial designs
While statistical sampling and analytical methodologies are proven to be highly effective in identifying patient subsets and trial effectiveness outcomes for RCTs for nonorphan drug trials, the same methodologies may not prove effective in the case of rare disease trial designs. It is important to identify efficient design and analysis techniques. While it is recommended to adopt well-defined, widely used, and regulatory-approved techniques, evaluations need to be performed and continuous monitoring is required to establish method validity and adaptability to various trial designs for rare diseases [ 3 ]. In studies of rare diseases, small sample sizes can severely restrict design options, hinder the usage of standard statistical models as mandated by regulators, and make replication of study models difficult. To assess the efficacy and safety of potential treatments, it is imperative to explore novel and innovative statistical designs [ 23 ]. It is also important to identify an appropriate observation population and study cohort to ensure that bias is properly addressed by using computer simulation models and advanced statistical techniques. While computer simulation methodologies can address the population issue to a considerable extent by identifying patient cohorts for randomized studies; it is also important to systematically study the interactions between treatment and disease phenotypes to prepare targeted trials to understand drug-patient interactions under personalized settings and individual requirements. Statistical algorithmic models can prove helpful in this regard. Digital endpoints generated from sensors installed in wearables can provide valuable real-time data that can be fed to computer simulation models to identify appropriate biomarkers and generate clinically relevant endpoints or surrogate endpoints [ 24 ]. However, there is a challenge, as identifying relevant and meaningful data from multiple observations in the form of digital data is time-consuming and requires multiple iterations.
Recommendations for improving the clinical trial design of rare diseases
Effective usage of natural history and disease registry data for trial design.
The strategic utilization of natural history and disease registry data plays a pivotal role in the design of clinical trials for rare diseases. This data serves as a roadmap, providing valuable insights into the complex and unique progression trajectories of these diseases, which is an essential component in assessing the impact of new therapeutic interventions [ 25 ]. It is important to harness the crucial information around these studies to derive quantifiable biomarkers to effectively chart the therapeutic roadmap, design the trial along with devising a robust drug development plan to augment the possibilities of regulatory approval supported by appropriate safety and efficacy data. Studies have been conducted on Duchenne Muscular Dystrophy based on natural history data for identification of relevant biomarkers based on progression and severity and these data provided valuable data for regulatory assessment [ 26 , 27 ]. It is to be noted that the landscape of natural history studies on rare diseases faces multiple challenges, including a limited pool of participants, issues with data quality, and the existence of data silos [ 28 ]. However, innovative solutions to design simulation models utilizing machine learning, are emerging to address these obstacles, fostering an environment of collaboration among stakeholders and promoting shared learning to enhance knowledge and expedite orphan drug discovery. Properly designed and maintained non-proprietary patient and disease registries based on real-world data, clinician and patient-reported outcomes and natural history study databases are cornerstones in this endeavor, offering a wealth of information about rare diseases [ 23 ]. These resources enable the design of robust clinical trials equipped with outcome measures that are both relevant and clinically meaningful. By harnessing this data, researchers can significantly enhance the design of clinical trials for rare diseases. This will lead to the development of more effective therapies in the EU, enable informed regulatory approvals, and pave the way for improved patient outcomes, marking a significant stride in developing and implementing a robust patient-centered approach toward orphan drug development.
Adoption of innovative clinical trial design
In clinical trial designs for orphan drugs, the process of obtaining approvals should take into account the outcome variations and underlying variability in disease manifestations. Due to the lack of a sufficient population, Phase 3 studies for orphan drug approvals tend to include a smaller number of patients, do not have placebo controls in many cases, and employ nonrandomized and unblinded trial designs, such as a single-arm design and surrogate endpoints, for assessing efficacy [ 3 ]. This requires proper planning, collaboration, and timeliness. As in most cases, rare disease trials involve multiple sites, and consensus is required between researchers and regulatory agencies in terms of the definition and classification of the disease under trial, identification of appropriate biomarkers and endpoints, and assessment of outcomes on commonly accepted standards. Under these circumstances, innovative trial designs such as basket trials and umbrella trials can help to address these issues. Basket trials enable researchers to investigate certain disease types with specific genetic biomarkers under a common trial design and protocol. Using this approach, researchers test the efficacy of targeted therapies by grouping patients based on molecular characteristics of the disease and provide valuable insights into the potential benefits of a treatment in rare diseases. These trials use a common targeted intervention. On the other hand, umbrella trials involve enrolling patients with the same disease but with different molecular or genetic subtypes. This design allows researchers to test multiple targeted therapies simultaneously within the same disease population, including rare diseases. Umbrella trials help identify which subgroups of patients may benefit from specific treatments, leading to more personalized and effective interventions. These trials use multiple targeted interventions. Both of these trials can also be designed using control groups through randomization, thus ensuring bias reduction and effective assessment of clinical outcomes. These trials have certain advantages, such as sharing the same control group to improve efficiency, reducing the likelihood of patients receiving a placebo, allowing comparisons between active substances and pooling of data from active treatments, and sharing of resources, thus leading to reduced trial costs and more efficient use of trial logistics [ 16 ]. In a rare disease setting, these trials may face some challenges around sponsor coordination in terms of multiple treatment trials, complex study design, competing and conflicting interests among stakeholders, operational challenges when international centers and multiple sites are involved, and implementing and following a common protocol. In this scenario, the ERICA, ERNs, patient advocacy groups, and centers of excellence can play an active role in identifying suitable patient populations and can act as co-ordinators or collaborators for designing, funding, and assisting in conducting trials through disease expertise, data sharing, execution management and assessing the outcomes of trials in multicenter settings [ 16 ].
Adaptive trial design for rare disease trials
Adaptive trial designs can offer flexibility and increase the efficacy of rare disease trials. Adaptive design trials encompass modified randomization procedures, the addition or discontinuation of treatment arms or doses, sample size adjustments based on interim results, adaptive patient population enrichment, and the incorporation of prespecified rules for efficacy. These designs in exploratory settings allow for the evaluation of various doses, regimens, and populations, focusing on the most favorable observations that will ensure promising results. They increase flexibility and acceptability and maximize the trial’s potential based on gathered data. Prespecified modifications maintain validity and integrity while adjusting elements of the study design [ 29 ]. The statistical approach of these trial designs enables modifications of study elements for minimizing errors, careful planning, and ensuring trial ethics and integrity [ 8 ]. While designing these trials, it is important to address and mitigate challenges around operational logistics, feasibility, and access to technical expertise. These are crucial considerations in designing clinical trials and special attention should be given to rare disease trials. Adequate study design expertise is necessary to ensure appropriate planning, for which experienced clinical researchers, statisticians, and healthcare professionals who are well-versed in rare diseases and orphan drug trial design, execution, and outcome assessment should be identified. Additionally, maintaining data and trial integrity becomes essential for post-interim analyses. This requires data storage and analytics planning. Addressing concerns related to bias in estimated treatment effects further strengthens the integrity of the trial, and outcomes and observations become more acceptable to regulators [ 8 ].
Clinical trials through inferentially seamless adaptive designs
In the adaptive seamless design, trials are merged, and analyses are seamlessly integrated by including data from patients enrolled both before and after the adaptation in the final analysis [ 30 ]. Adaptive seamless designs, particularly in the context of rare diseases, offer an appealing approach when traditional group sequential designs for assessing efficacy or futility may not be feasible due to limited sample sizes [ 31 ]. These designs integrate a Phase 2 study, which focuses on treatment selection, with a Phase 3 study for confirmatory testing. This integration allows for treatment selection and the re-evaluation of sample size at a predefined interim analysis [ 3 ]. The use of adaptive seamless designs in rare disease clinical trials offers several benefits. It maximizes patient data utilization, leading to stronger conclusions, while reducing the number of patients and saving time and costs in Phase 3. It improves target dose and participant selection, explores covariates between Phase 2 endpoints and Phase 3 outcomes, and provides valuable information on treatment effects and safety by following patients from terminated treatment groups. Additionally, it allows for treatment modifications, enhancing the chances of patients receiving safe and effective treatments [ 32 ]. Challenges arise when working with these designs, including the time required for their design and the need for appropriate analyses to account for potential bias in treatment effect estimates due to data combinations at different study phases [ 16 ].
Decentralized clinical trial design for rare diseases
Decentralized methods involve conducting assessments in alternative locations such as participants’ homes, local clinics, or digital platforms on mobile devices or computers and not at centralized medical facilities. Decentralized clinical trial (DCT) approaches that incorporate physical and virtual consultations, along with online access to medicines or providing drugs through local clinicians or pharmacists, can bring substantial benefits by reducing the burden on patients and their families [ 33 ]. DCTs do not completely remove the physical interactions between clinicians and patients. It leverages digital health technologies (DHTs) such as medical devices and wearables. for electronic collection and usage of reliable diagnostic and clinical data, clinical outcome assessments (COAs), Patient and Observer Reported Outcomes, and clinical health records. These enable clinicians to determine exploratory patient-relevant endpoints, enable targeted patient recruitment, and help in collecting and updating data registries for secondary usage in future trials as reference points. This integrated approach will help clinical trial design be more focused on the outcomes. DCTs have their setup challenges in terms of technology adoption and usage, data privacy and technology literacy concerns, and site readiness with appropriate infrastructure, trained personnel, and logistical support. Keeping in mind the needs of patients, industry and clinical stakeholders need to upgrade technical aspects and knowledge of data collection and analysis [ 34 ]. Addressing data privacy concerns while sharing data across multiple trial sites as per domestic and international regulations will ensure confidentiality and appropriate usage of data. If the challenges are properly addressed, DCTs can play a crucial role in terms of increased adoption in conducting rare disease clinical trials and acceptance across regulators during decision-making.
Usage of external controls in designing effective rare disease trials
As rare disease clinical trials are subjected to small patient populations, nonrandomization has been adopted in multiple scenarios. Nonrandomized clinical trials that compare against external controls have proven effective in an expanding range of cases, especially in the context of rare diseases. These designs are particularly valuable when randomization is impractical or ethically challenging, or when the available pool of patients with a specific condition is limited [ 22 ]. Clinical trial researchers need to understand disease progression and interpret measurements accordingly, but precise methods often don’t translate well to real-world practice. Control groups in these studies are generally being replaced with historical controls based on natural history data. Natural history studies provide a context for a “dry run” of clinical trials, facilitate biological endpoint selection, help in designing an informed clinical trial program with appropriate inclusion/exclusion criteria, help in selecting proper biomarkers for treatment delivery [ 28 ], and help to understand long-term trial issues. Errors in this stage are less costly and can inform improvements in the actual trial. Clinical assessments are performed accordingly under small patient population settings providing contextual evidence for regulatory assessment. Incorporating external controls in clinical trials requires meticulous analysis and adept adjustment. Controls are chosen from data obtained from registries, medical records, and scientific literature. Data are also obtained from data from expanded access programs, which provide access to investigational treatments for patients with serious or life-threatening conditions [ 22 ]. The process of utilizing external controls entails rigorous statistical methods that help minimize potential biases that can influence results and ensure the validity and reliability of the trial findings.
Protocol flexibility for conducting rare disease clinical trials
In many situations, regulators have provided flexibility to sponsors and trial organizers for rare disease clinical trials. Placebo control can be omitted, trial design can be unblinded and nonrandomized, surrogate endpoints can be used for efficacy assessment and trials can be single-arm [ 3 ]. Another possible approach is the substitution of clinical endpoints with biomarkers, ideally in the form of a panel of biomarkers representing various aspects of the disease [ 28 ] In the case of rare diseases with insufficient biomarkers, the totality of trends in clinical efficacy and safety data can be assessed by utilizing the entire body of available evidence followed by response simulations from clinical trials and pharmacological modeling of data based on reliable biomarkers [ 25 ]. These enable adjusting sample sizes, treatment arms, or endpoints, to ensure the most efficient use of resources and maximize the chances of obtaining meaningful results. The preference or requirement for evidence about safety from RCTs must be still present, as these are based on feasibility considerations, such as the practicality of measuring and monitoring safety levels. However, small population trials may not provide sufficient information about safety or efficacy in the long term. To address the unmet medical needs of patients and prioritize public health, it may be possible to consider granting marketing authorizations with less comprehensive data than typically needed [ 8 ]. Hence, ongoing monitoring and data collection of a new medicine is being proposed for evaluating efficacy endpoints. It involves monitoring safety, minimizing risks, conducting additional studies for more knowledge, and evaluating risk-minimization strategies. This helps gather valuable insights to enhance understanding and management of the medicine’s benefits and risks [ 31 ]. Regulators can provide the flexibility to sponsors to devise a plan for data monitoring and continuous feedback rather than solely relying on trial outcomes. Additionally, protocol flexibility facilitates the inclusion of novel trial methodologies, such as basket or umbrella trials, which can evaluate multiple treatments or subgroups within the rare disease population simultaneously [ 35 ]. By embracing protocol flexibility, researchers can address rare disease trial-specific challenges, apply statistical models for design and outcome analysis that are tailored to adaptive trial settings, and improve the chances of successful outcomes and the development of effective therapies for patients with rare diseases. Another aspect regarding adopting flexible protocol is to undertake a multi-faceted approach to train assessors in data evaluation for rare diseases, to enable them to approve applications based on limited data. This includes a deeper understanding of disease heterogeneity, an understanding of the uniqueness of disease manifestation through direct patient interaction, the adoption of machine learning and iterative approaches in data assessment, and ongoing skill enhancement programs. Specific training programs in line with the National Institute of Health (NIH) funded R25 Rare Disease Clinical Research Training Program can impart specialized skills to assessors in rare disease clinical data assessment. To promote innovation in clinical trial design, the Scientific Advice Working Party (SAWP) under the Committee for Medicinal Products for Human Use (CHMP) of the EMA administers a process to evaluate and qualify innovative methodologies for drug development. This involves providing scientific advice and opinions on various methodologies, such as the multiple comparison procedure modeling, which is recognized as an effective statistical approach for model-based design and analysis [ 8 ].
Data extrapolation for efficacy assessment and re-usage of existing treatment
Data extrapolation can be adopted by researchers by leveraging available data from various sources. This can include using historical control data, real-world evidence, registries, electronic health records, clinical literature, and scientific papers or data from similar diseases or patient populations and applying extrapolation techniques to infer the treatment’s effectiveness. By extrapolating data, researchers can make informed judgments about the efficacy of the treatment in the context of the rare disease [ 36 ]. This has been utilized to extend existing treatments in adults to the pediatric population based on scientific evidence and assessing their efficacy. This strategy was adopted as part of a need to address the practical and ethical challenges associated with conducting clinical trials in pediatric patient populations and was discussed in an EMA concept paper. If there are scientifically robust data available for an orphan indication, it might be possible to extend these data to offer substantiating evidence for the reasonable certainty of effectiveness, likely advantage, and safety for another orphan indication or subset of a population. The determination of the amount and reliability of data to be utilized for extrapolation, along with the timing of the extrapolation (whether in early or late phase trials), must be carefully assessed on an individual basis for each case [ 16 ]. Data can be obtained from clinical trials, real-world evidence, and non-clinical studies. Early identification of relevant data, in collaboration with regulatory authorities, is crucial and can be aligned with a pediatric investigation plan development during initiating studies in adults [ 23 ]. As there is limited experience and educational gap in this area thus far, it is important to assess all the nuances and plan the methodologies and validation models by applying rigorous statistical and scientific methods to ensure the correctness and reliability of the extrapolated results. To address knowledge gaps and skills, it is crucial to implement a skill development initiative and continuous assessment for biostatisticians, clinicians, and data analysts.
Engagement of patients during study design
It is important to engage the currently known patient population through patient advocacy groups, outreach programs, and advertisements while designing clinical trials. Engaging patients at an early stage and continuous feedback and information sharing can help understand the concerns of the relevant group and can streamline various aspects of trial design, such as safety considerations, benefit-risk assessment, and selection of endpoints [ 16 ]. It is recommended to consult with patients who have experience with clinical trials, and early engagement is preferable. Study designs that are based on patient preferences can serve as crucial and valuable reference points in the overall drug development process and regulatory decision-making. Patient motivation also plays a crucial role, as any new treatment that can prove to be effective will have increased interest among target groups and continuous engagement gives a sense of responsibility and belonging to the overall process [ 5 ]. Patient participation and retention are very crucial in conducting successful orphan drug trials, especially in countries with limited patient populations. Collaboration across international centers, engagement with patient advocacy groups, involvement of specialist centers, and proper patient education by research staff is essential for successful participation [ 8 ].
Use of Real-World Data in rare disease trial design
Clinical trials face numerous challenges that hinder the timely delivery of promising treatments to patients. However, the use of real-world data (RWD) is emerging as a tool to improve the pace of therapy development. RWD, collected during routine patient care, patient-reported outcomes, handwritten notes, medical records, charts, electronic health records, registry data, and observer reports is gaining support from regulators and can be applied in three key use cases: synthetic control arms (SCAs) to replace or augment standard control arms in trials, precision registries for adaptive trial design, and clinical trial site feasibility to improve patient enrollment. SCAs based on a patient’s standard of care (SOC), in particular, offer an alternative to traditional control arms, which may be unethical in certain cases and help in the identification of endpoints that may vary based on the patient’s disease condition [ 37 ]. RWD also enables researchers to optimize study design, assess site feasibility based on patient demographic information of the site, collect more comprehensive and diverse data, and overcome challenges associated with data quality through the application of statistical methods and machine learning algorithms [ 38 ]. The integration of RWD in clinical trials holds promise for advancing the development of therapies by quantifying benefits or risks, thereby increasing the success probability and improving health outcomes for patients [ 23 ]. The key challenges lie in ensuring data quality, reliability, and interoperability. As many RWDs are extracted through electronic means there are chances of missing data points, and the quality of data may become compromised when merging with multiple other sources for analysis purposes. It is important to classify the data based on type and source and apply appropriate extraction and transformation procedures before they are analyzed for decision-making. To improve interoperability and to ensure data privacy, tokenizing clinical data will be helpful enabling intersystem operability, reliability of information, and availability and accessibility as and when needed [ 39 ]. RWD leads to the generation of appropriate RWEs that enable hybrid study methodologies combining the strengths of RCT studies with RWE such as RWE-RE (Real World Evidence-Randomized Enrichment design), mitigating limitations and potentially enhancing or replacing RCTs in many cases, thus providing a patient-centric approach towards statistical data analysis and supporting regulatory decision making [ 23 ].
Some steps taken by the International Council on Harmonization (ICH) are to update the E8 and E6 guidelines to include more flexible study designs and diverse data sources. This includes discussions on pragmatic study designs and guidance on using RWD in conjunction with or as a substitute for traditional data collection. The Professional Society for Health Economics and Outcomes Research [ 40 ]–International Society for Pharmacoepidemiology [ 41 ] (ISPOR-ISPE) Special Task Force has also provided recommendations to enhance clinical study practices and improve the acceptance of RWE by regulatory authorities, emphasizing good practices, transparency, and overall study conduct [ 42 ]. As expertise grows, researchers, clinicians, and regulators are likely to adopt and apply these designs on a larger scale [ 22 ]. Since the publication of the Operational, Technical, and Methodological (OPTIMAL) framework in 2019, there certain initiatives to incorporate selective usage of RWE for informing regulatory decisions by EMA.
Innovative approach to drug development planning
The development planning of rare disease trials necessitates a unique approach. Most of the companies still adhere to traditional clinical pharmacology approaches, which may not be as effective for rare diseases due to their unique characteristics and the small patient populations involved [ 43 , 44 ]. As a result, many companies are adopting the oncological approach, where the product is tested in Phase I, particularly in patients with high morbidity or mortality. This approach allows the enrolment of a substantial number of patients and enables early assessment of the product’s safety and efficacy. This further helps in the reduction of overall trial costs and helps in resource optimization [ 45 ]. The data findings facilitate Model Informed Drug Development (MIDD), to enable addressing gaps in pharmacological studies and provide sufficient clinical data to initiate later phases of trials. This helps in better clinical evaluation and facilitates dose adjustments as well as endpoint modifications across different patient subpopulations. This approach also ensures that high-risk patients are not subjected to exclusion based on randomization and receive the treatment as part of the standard of care procedures [ 44 , 45 ].
Small to medium-sized enterprises (SMEs) are integral to the development and distribution of treatments for rare diseases. These companies play a crucial role in optimizing logistics and leveraging expertise [ 46 ]. They often enter into commercial agreements with larger companies, facilitating initial developmental activities of the target therapy, performing an initial assessment of the product’s safety and efficacy, and transferring the same to larger entities for carrying out later activities. Through collaborative efforts, SMEs can access the resources and knowledge of larger companies, potentially accelerating the development and approval of treatments efficiently and effectively [ 47 , 48 ]. This collaborative model can help to overcome the challenges associated with developing treatments for rare diseases, facilitating reduced timelines, and optimizing the financial and resource burdens, ultimately benefiting patients who may not have access to these life-saving therapies otherwise [ 47 , 48 ].
Clinical trial research initiatives
Under the Seventh Framework Program (FP7), the European Union [ 2 ] funded three research projects to identify innovative trial designs for rare diseases. The challenges around small patient populations and heterogenous endpoints are recognized by researchers, industry, and regulators and these initiatives aimed to address these challenges and develop effective study methodologies and strategies to address these challenges. The studies aimed to improve the statistical methodology employed in trials involving a small number of patients through enhanced focus on the integration of trial design, conduct methodology, and outcomes and endpoint analysis. This approach ensures a comprehensive perspective and leads to refined methodologies that optimize the overall trial process [ 8 ]. The three projects have contributed to the implementation of study models and frameworks that are being increasingly adopted by researchers and sponsors to conduct rare disease clinical trials. Regulators have also shown increasing acceptance of novel methodologies and incorporating the findings in orphan drug approvals. More such initiatives focusing on specific therapeutic areas in coordination with ERNs will help in the development of other design methodologies that may address time criticality, can address endpoint adjustments, and modify the design based on continuous patient feedback. One key initiative that can be taken is a program similar to the Rare Disease Endpoint Advancement Program (RDEA) [ 49 ] by the US Food and Drug Administration (FDA). This is a dedicated program to advance the development of endpoints for clinical trials in rare diseases. This can be an EC-funded program under the supervision of EMA in collaboration with researchers, sponsors, and other regulatory authorities focusing on identifying and creating innovative, meaningful, and reliable endpoints to effectively assess rare disease treatment effectiveness. The EC-funded projects are listed below in Table 2 :
The development of effective treatments for rare diseases remains a significant challenge due to the low prevalence and unique characteristics of these conditions. The challenges faced in clinical trials for orphan drugs in the EU are multifaceted and include patient recruitment, disease heterogeneity, limited knowledge of the natural history of rare diseases, lack of existing clinical study data, variability in disease characteristics, ethical considerations, cost challenges, and regulatory hurdles. Despite various financial incentives and grants extended to sponsors and researchers for rare disease research, all these challenges prove to be significant roadblocks in drug development as efficacy assessment becomes difficult due to the lack of test subjects and insufficient data that does not give enough evidence in favor of the medicine. Hence, close attention is required on an ongoing basis to address these issues. Due to the unique nature of rare diseases, there may not be a fit-for-all approach and traditional randomized trial methodologies prove ineffective in different settings. To mitigate these challenges, a few steps have been and can be taken, including the establishment of unified patient databases, PROMs, and RWE generation that has provided and has further potential to provide valuable information for drug development. Innovative approaches to clinical trial design, such as adaptive trial designs, basket and umbrella trials, and the use of real-world evidence, are being explored to address the challenges associated with rare disease trials. Apart from these, by embracing innovative approaches to drug development planning, utilizing external natural history study-based controls, using statistical modeling based on registry, standard of care, and natural history data, ensuring protocol flexibility, engaging patients, and incorporating RWD, researchers can overcome the unique challenges of rare disease trials and improve the development of effective therapies for patients with rare diseases. Continued collaboration between stakeholders, including researchers, clinicians, regulators, patient advocacy groups, and industry is required to foster continuous innovation and the proposed approaches aim to improve the efficiency, effectiveness, and generalizability of clinical research in the context of rare disease patient populations.
Data availability
All data used for analyzing the clinical trial landscape were accessed from the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization. The data and related information are publicly available at: https://trialsearch.who.int/ . All information generated during this study is presented and documented within the article itself. The data used and analyzed in the study is publicly available in Supplementary File 2. This includes all the necessary information and relevant data points used in the study.
Abbreviations
Committee for Medicinal Products for Human Use
Clinical Outcome Assessment
Decentralized Clinical Trial
Digital Health Technology
European Commission
European Joint Program on Rare Diseases (EJP-RD)
European Medicines Agency
European Rare Disease Registry Infrastructure
European Rare Disease Research Coordination and Support Action Consortium
European Reference Network
European Union
European Confederation of Pharmaceutical Entrepreneurs
European Organization for Rare Diseases
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
International Rare Diseases Research Consortium
Professional Society for Health Economics and Outcomes Research–International Society for Pharmacoepidemiology
National Institute of Health
Operational, Technical, and Methodological Framework
Patient Reported Outcome Measures
Randomized Controlled Trial
Rare Disease Endpoint Advancement Program
Real World Data
Real World Evidence
Real World Evidence– Randomized Enrichment
Scientific Advice Working Party
Synthetic Control Arm
Standard of Care
US Food and Drug Administration
Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical trials in small population [Internet]. European Medicines Agency. United Kingdom: European Medicines Agency; 2006 [cited 2023 May 24]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-trials-small-populations_en.pdf .
Talko M, Caudet L, European Commission. 2016 [cited 2023 Feb 16]. 7th Framework Programme for Research. https://ec.europa.eu/commission/presscorner/detail/en/MEMO_16_146 .
Hilgers R. Design and analysis of clinical trials for small rare disease populations. J Rare Dis Res Treat. 2016;1(3):53–60.
Article Google Scholar
Murphy MF, Applied Clinical T. 2016 [cited 2023 Apr 13]. Rare Diseases: Meeting the Unique Challenges of Orphan Drug Development. https://www.appliedclinicaltrialsonline.com/view/rare-diseases-meeting-unique-challenges-orphan-drug-development .
Motte S. de la. Synteract HCR. 2018 [cited 2023 Apr 20]. Orphan Indications and Clinical Trials - Why Rare Diseases Warrant Special Treatment. https://www.clinicalleader.com/doc/orphan-indications-and-clinical-trials-why-rare-diseases-warrant-special-treatment-0001 .
ProRelix R. ProRelix Research. 2022 [cited 2023 Mar 31]. Planning a Rare Diseases Clinical Trial? View from United States and Europe. https://prorelixresearch.com/rare-disease-clinical-trial-in-us-europe-prorelix-research/ .
Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–83.
O’Connor DJ, Hemmings RJ. Coping with small populations of patients in clinical trials. Expert Opin Orphan Drugs [Internet]. 2014;2(8):765–8. http://www.tandfonline.com/doi/full/ https://doi.org/10.1517/21678707.2014.931221 .
Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9(1):170.
Article PubMed PubMed Central Google Scholar
Ndebele P, Blanchard-Horan C, Shahkolahi A, Sanne I. Regulatory challenges Associated with conducting Multicountry clinical trials in resource-limited settings. JAIDS J Acquir Immune Defic Syndr. 2014;65(Supplement 1):S29–31.
Article PubMed Google Scholar
McCormack P, Woods S, Aartsma-Rus A, Hagger L, Herczegfalvi A, Heslop E et al. Guidance in Social and Ethical Issues Related to Clinical, Diagnostic Care and Novel Therapies for Hereditary Neuromuscular Rare Diseases: Translating the Translational. PLoS Curr [Internet]. 2013; https://currents.plos.org/md/index.html%3Fp=2561.html .
ICTRP Secretariat. World Health Organization. 2018 [cited 2023 May 14]. International Clinical Trials Registry Platform (ICTRP). https://trialsearch.who.int/ .
EJP RD INSERM. EJP-RD. [cited 2023 Mar 6]. European Joint Programme on Rare Diseases. https://www.ejprarediseases.org/ .
EUCOPE. EUCOPE. 2017 [cited 2023 Mar 9]. European Confederation of Pharmaceutical Entrepreneurs (EUCOPE). https://www.eucope.org/ .
The Scientific Secretariat of IRDiRC [Internet]. 2022 [cited 2023 Feb 12]. International Rare Diseases Research Consortium. https://irdirc.org/ .
MAPI RESEARCH TRUST, MAPI RESEARCH TRUST. 2002 [cited 2023 Feb 20]. PROQOLID. https://eprovide.mapi-trust.org/about/about-proqolid#title-0 .
ERICA Administrator. European Rare Disease Research Coordination and Support Action consortium (ERICA). 2022 [cited 2023 May 28]. WP3 Patient-Centred Research, ERICA. https://erica-rd.eu/work-packages/patient-centred-research/ .
EURORDIS. EURORDIS. 2022 [cited 2023 May 23]. EURORDIS-Rare Diseases Europe. Proposal on the Revision of Orphan Medicinal Products and Paediatric Regulation. https://www.eurordis.org/publications/eurordis-proposal-on-the-revision-of-the-orphan-medicinal-products-and-paediatric-regulation/ .
Day S, Jonker AH, Lau LPL, Hilgers RD, Irony I, Larsson K, et al. Recommendations for the design of small population clinical trials. Orphanet J Rare Dis. 2018;13(1):195.
Mellerio JE. The challenges of clinical trials in rare diseases. Br J Dermatol. 2022;187(4):453–4.
Rare Daily Staff. Global Genes. 2021 [cited 2024 Feb 5]. Bluebird Withdraws Gene Therapies in Europe as it Winds Down Operations There. https://globalgenes.org/raredaily/bluebird-withdraws-gene-therapies-in-europe-as-it-winds-down-operations-there/ .
Baumfeld Andre E, Reynolds R, Caubel P, Azoulay L, Dreyer NA. Trial designs using real-world data: the changing landscape of the regulatory approval process. Pharmacoepidemiol Drug Saf. 2020;29(10):1201–12.
Mulberg AE, Bucci-Rechtweg C, Giuliano J, Jacoby D, Johnson FK, Liu Q, et al. Regulatory strategies for rare diseases under current global regulatory statutes: a discussion with stakeholders. Orphanet J Rare Dis. 2019;14(1):36.
Dreghici RD, Morris C, Servais L, Clinical L. 2023 [cited 2023 Jun 1]. New Advancement Toward The First Primary Endpoint Qualification Of A Digital Endpoint For Duchenne. https://www.clinicalleader.com/doc/new-advancement-toward-the-first-primary-endpoint-qualification-of-a-digital-endpoint-for-duchenne-0001 .
Manolis E, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br J Clin Pharmacol. 2009;68(4):493–501.
Cox GF. The art and science of choosing efficacy endpoints for rare disease clinical trials. Am J Med Genet A. 2018;176(4):759–72.
Crow RA, Hart KA, McDermott MP, Tawil R, Martens WB, Herr BE, et al. A checklist for clinical trials in rare disease: obstacles and anticipatory actions—lessons learned from the FOR-DMD trial. Trials. 2018;19(1):291.
Kakkis ED, O’Donovan M, Cox G, Hayes M, Goodsaid F, Tandon P, et al. Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J Rare Dis. 2015;10(1):16.
Potgieter D, Kilian L, Bradham W, Merative US LP. 2023 [cited 2023 Aug 9]. Adaptive Clinical Trial Success Via A Unified Data Management And Acquisition Platform. https://www.merative.com/content/dam/merative/documents/white-paper/adaptive-clinical-trial-success-via-a-unified-data-management-and-acquisition-platform.pdf .
Maca J, Bhattacharya S, Dragalin V, Gallo P, Krams M. Adaptive seamless phase II/III Designs—Background, operational aspects, and examples. Drug Inf J. 2006;40(4):463–73.
Hilgers RD, Bogdan M, Burman CF, Dette H, Karlsson M, König F, et al. Lessons learned from IDeAl — 33 recommendations from the IDeAl-net about design and analysis of small population clinical trials. Orphanet J Rare Dis. 2018;13(1):77.
Eisenbeisz E, Omega S. 2018 [cited 2023 Jun 6]. Adaptive Seamless Design For Phase 2/3 Studies: Basic Concepts & Considerations. https://www.clinicalleader.com/doc/adaptive-seamless-design-for-phase-studies-basic-concepts-considerations-0001 .
Moore J, Goodson N, Wicks P, Reites J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J Rare Dis. 2022;17(1):240.
Article CAS PubMed PubMed Central Google Scholar
Haenel EK. 2022 [cited 2023 May 26]. Key Considerations For The Implementation Of Successful Decentralized Clinical Trials. https://www.clinicalleader.com/doc/key-considerations-for-the-implementation-of-successful-decentralized-clinical-trials-0001 .
Ekangaki A, Premier R. 2023 [cited 2023 May 26]. Can Your Protocol Flex? The Importance Of Adaptive Trial Designs In Precision Oncology Studies. https://www.clinicalleader.com/doc/can-your-protocol-flex-the-importance-of-adaptive-trial-designs-in-precision-oncology-studies-0001 .
Biostatistics WP, Modelling and, Party SW, Party PW. Scientific Advice Working Party. Use of extrapolation in the development of medicines for paediatrics [Internet]. European Medicines Agency. London: European Medicines Agency; 2018 [cited 2023 Jun 6]. https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-reflection-paper-use-extrapolation-development-medicines-paediatrics-revision-1_en.pdf .
O’Connor D, Ciox Life S. 2021 [cited 2023 May 26]. Real-World Data Enhanced Clinical Trial Design. https://www.clinicalleader.com/doc/real-world-data-enhanced-clinical-trial-design-0001 .
Loo EF. 2023 [cited 2023 May 26]. Speed Up, Collect More, And Reach Further: Using RWD To Optimize Your Clinical Trials. https://www.clinicalleader.com/doc/speed-up-collect-more-and-reach-further-using-rwd-to-optimize-your-clinical-trials-0001 .
Myles J, Kamau A. Decentralized Trial Research Alliance (DTRA),Ikaika Health LLC. 2022 [cited 2023 May 26]. Using RWE In Clinical Trials: Challenges & Opportunities. https://www.clinicalleader.com/doc/using-rwe-in-clinical-trials-challenges-opportunities-0001 .
ISPOR. The Professional Society for Health Economics and Outcomes Research. 2018 [cited 2023 May 18]. The Professional Society for Health Economics and Outcomes Research. https://www.ispor.org/heor-resources/about-heor .
INTERNATIONAL SOCIETY FOR PHARMACOEPIDEMIOLOGY [Internet]. [cited 2023 Jun 1]. International Society for Pharmacoepidemiology. https://www.pharmacoepi.org/ .
Burns L, Roux N, Le, Kalesnik-Orszulak R, Christian J, Hukkelhoven M, Rockhold F, et al. Real-world evidence for Regulatory Decision-Making: Guidance from around the World. Clin Ther. 2022;44(3):420–37.
Ghadessi M, Di J, Wang C, Toyoizumi K, Shao N, Mei C, et al. Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet J Rare Dis. 2023;18(1):79.
Mitra A, Lee JB, Steinbach D, Hazra A, Krishna R. Rare oncology therapeutics: review of clinical pharmacology package of drug approvals (2019–2023) by US FDA, best practices and recommendations. J Pharmacokinet Pharmacodyn [Internet]. 2023 Dec 4 [cited 2024 Feb 9];50(6):475–93. https://link.springer.com/article/ https://doi.org/10.1007/s10928-023-09896-2 .
Iasonos A, O’Quigley J. Randomised phase 1 clinical trials in oncology. Br J Cancer. 2021;125(7):920–6.
Rollet P, Lemoine A, Dunoyer M. Sustainable rare diseases business and drug access: no time for misconceptions. Orphanet J Rare Dis. 2013;8(1):109.
Ascher J, M’lika A, Graf J, Prabhakaran M, McKinsey. & Company. 2021 [cited 2024 Feb 9]. Successful launches in rare diseases. https://www.mckinsey.com/~/media/McKinsey/Industries/Pharmaceuticals%20and%20Medical%20Products/Our%20Insights/How%20to%20successfully%20launch%20a%20rare%20disease%20drug%20in%20a%20patient%20centric%20world/Successful-launches-in-rare-diseases-vFinal.pdf
Alfano S, Amberg C, Peters N, Salazar P, Vieux-Rochas M, Welton S, McKinsey. & Company. 2023 [cited 2024 Feb 9]. Treating rare diseases: How digital technologies can drive innovation. https://www.mckinsey.com/industries/life-sciences/our-insights/treating-rare-diseases-how-digital-technologies-can-drive-innovation
Center for Drug Evaluation and Research. U.S. Food and Drug Administration. 2022 [cited 2022 Oct 29]. Rare Disease Endpoint Advancement Pilot Program. https://www.fda.gov/drugs/development-resources/rare-disease-endpoint-advancement-pilot-program .
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Integrated Design and Analysis of clinical trials in SPG (IDeAl). https://cordis.europa.eu/project/id/602552/reporting .
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Innovative methodology for small populations research. https://cordis.europa.eu/project/id/602144 .
Baran-Kooiker A, Czech M, Kooiker C, OVERVIEW OF REGULATORY, INITIATIVES IN THE EUROPEAN UNION TO STIMULATE RESEARCH AND ACCELERATE ACCESS TO ORPHAN DRUGS AND OTHER HIGH MEDICAL NEED PRODUCTS. Acta Pol Pharm - Drug Res. 2019;76(1):3–17.
Google Scholar
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Advances in Small Trials Design for Regulatory Innovation and Excellence. https://cordis.europa.eu/project/id/603160 .
Download references
Acknowledgements
Not applicable.
No Funding was received for this work.
Author information
Authors and affiliations.
Dept. of Pharmaceutics, Centre of Excellence in Regulatory Sciences, JSS College of Pharmacy, JSS Academy of Higher Education and Research, SS Nagara, Mysore, Karnataka, 570015, India
Sangita Mishra & MP Venkatesh
Faculty of Pharmaceutical Sciences, UCSI University, Kuala Lumpur, Malaysia
MP Venkatesh
You can also search for this author in PubMed Google Scholar
Contributions
MP was responsible for conceptualizing the study and content. SM was responsible for data collection, analysis, and drafting of the manuscript.
Corresponding author
Correspondence to MP Venkatesh .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mishra, S., Venkatesh, M. Rare disease clinical trials in the European Union: navigating regulatory and clinical challenges. Orphanet J Rare Dis 19 , 285 (2024). https://doi.org/10.1186/s13023-024-03146-5
Download citation
Received : 28 June 2023
Accepted : 24 March 2024
Published : 31 July 2024
DOI : https://doi.org/10.1186/s13023-024-03146-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rare diseases
- Orphan drugs
- Clinical trials
- Novel methodologies
- Umbrella trials
- Basket trials
- Adaptive trials
- Decentralized trials
- Real world evidence
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
A literature review is an integrated analysis-- not just a summary-- of scholarly writings and other relevant evidence related directly to your research question.That is, it represents a synthesis of the evidence that provides background information on your topic and shows a association between the evidence and your research question.
Conducting a literature review is usually recursive, meaning that somewhere along the way, you'll find yourself repeating steps out-of-order. That is actually a good sign. Reviewing the research should lead to more research questions and those questions will likely lead you to either revise your initial research question or go back and find ...
The Literature Review by Diana Ridley The Literature Review is a step-by-step guide to conducting a literature search and writing up the literature review chapter in Masters dissertations and in Ph.D. and professional doctorate theses. The author provides strategies for reading, conducting searches, organizing information and writing the review.
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Writing a Literature Review. A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels ...
Steps for Conducting a Lit Review. 1. Choose a topic. Define your research question. 2. Decide on the scope of your review. 3. Select the databases you will use to conduct your searches. 4. Conduct your searches and find the literature. Keep track of your searches! 5. Review the literature. Finding "The Literature" Organizing/Writing
Conducting a literature review involves using research databases to identify materials that cover or are related in some sense to the research topic. In some cases the research topic may be so original in its scope that no one has done anything exactly like it, so research that is at least similar or related will provide source material for the ...
Abstract. Performing a literature review is a critical first step in research to understanding the state-of-the-art and identifying gaps and challenges in the field. A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in ...
As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries." Taylor, D. The literature review: A few tips on conducting it. University ...
Tips: Review the abstracts of research studies carefully. This will save you time. Write down the searches you conduct in each database so that you may duplicate them if you need to later (or avoid dead-end searches that you'd forgotten you'd already tried).; Use the bibliographies and references of research studies you find to locate others.
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information.
Literature reviews take time. Here is some general information to know before you start. VIDEO -- This video is a great overview of the entire process. (2020; North Carolina State University Libraries) --The transcript is included. --This is for everyone; ignore the mention of "graduate students". --9.5 minutes, and every second is important.
A literature review, also called a review article or review of literature, surveys the existing research on a topic. ... Steps for Writing a Literature Review. 1. Identify and define the topic that you will be reviewing. ... Conduct a Literature Search. Use a range of keywords to search databases such as PsycINFO and any others that may contain ...
lls the reader, and why it is necessary.3.2 Evaluate the nine basic steps taken to wr. te a well-constructed literature review.3.3 Conduct an electronic search using terms, phrases, Boolean operators, and filters.3.4 Evaluate and identify the parts of an empirical research journal article, and use that kn.
In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your ...
The Literature Review portion of a scholarly article is usually close to the beginning. It often follows the introduction, or may be combined with the introduction.The writer may discuss his or her research question first, or may choose to explain it while surveying previous literature.. If you are lucky, there will be a section heading that includes "literature review".
Step 3: Critically analyze the literature. Key to your literature review is a critical analysis of the literature collected around your topic. The analysis will explore relationships, major themes, and any critical gaps in the research expressed in the work. Read and summarize each source with an eye toward analyzing authority, currency ...
The main objectives of this chapter are fourfold: (a) to provide an overview of the major steps and activities involved in conducting a stand-alone literature review; (b) to describe and contrast the different types of review articles that can contribute to the eHealth knowledge base; (c) to illustrate each review type with one or two examples ...
In order to understand your topic, before you conduct your research, it is extremely important to immerse yourself in the research that has been done on your topic and the topics that might be adjacent to your particular research interest or questions. "a researcher cannot perform significant research without first understanding the literature in the field" (Boote & Beile, 2005, p. 3).
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
Since the literature review forms the backbone of your research, writing a clear and thorough review is essential. The steps below will help you do so: 1. Search for relevant information and findings. In research, information published on a given subject is called "literature" or "background literature.".
CONDUCTING YOUR LITERATURE REVIEW. 6. produce a reliable and unbiased summary of the existing research. This book will walk you through those steps one by one. Each chapter targets a specific part or stage in the literature review. Throughout this book, the elements and reporting structure of a systematic review serve as a framework for ...
• Writing the review • References literature {Table 2). The first step involves identifying the subject ofthe literature review. The researcher undertaking a quantitative study may have decided this already. However, for the individual undertaking a non-research based literature review this will be the first step. Selecting a review topic
Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers ... West CP (2012) Conducting systematic reviews in medical education: a stepwise approach ... (2008) The literature review: a step-by-step guide for students. London: SAGE. 22. Kelleher C, Wagener T (2011) Ten ...
Conducting a literature review . Conducting a literature review is an essential step in research that involves reviewing and analyzing existing literature on a specific topic. It's important to know how to do a literature review effectively, so here are the steps to follow: 1 . Choose a Topic and Define the Research Question:
When seeking information for a literature review or for any purpose, it helps to understand information-seeking as a process that you can follow. 5 Each of the six (6) steps has its own section in this web page with more detail. Do (and re-do) the following six steps: 1. Define your topic.
Describe the rationale for the review in the context of what is already known. Explain why the review questions or objectives lend themselves to a scoping review approach. PRISMA-ScR . This step outlines the overarching goals of the scoping review. It explains the rationale behind conducting the review and what the reviewers aim to achieve.
Method details Overview. A Systematic Literature Review (SLR) is a research methodology to collect, identify, and critically analyze the available research studies (e.g., articles, conference proceedings, books, dissertations) through a systematic procedure [12].An SLR updates the reader with current literature about a subject [6].The goal is to review critical points of current knowledge on a ...
This study employs a review of various literature types encompassing review papers, concept papers, points of view, empirical researchers, policy guidelines, and expert reviews to explore the challenges faced by researchers in conducting rare disease trials in the EU as well as patient perspectives, and regulatory hurdles.