Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 May 2023

Child and adolescent obesity
- Natalie B. Lister ORCID: orcid.org/0000-0002-9148-8632 1 , 2 ,
- Louise A. Baur ORCID: orcid.org/0000-0002-4521-9482 1 , 3 , 4 ,
- Janine F. Felix 5 , 6 ,
- Andrew J. Hill ORCID: orcid.org/0000-0003-3192-0427 7 ,
- Claude Marcus ORCID: orcid.org/0000-0003-0890-2650 8 ,
- Thomas Reinehr ORCID: orcid.org/0000-0002-4351-1834 9 ,
- Carolyn Summerbell 10 &
- Martin Wabitsch ORCID: orcid.org/0000-0001-6795-8430 11
Nature Reviews Disease Primers volume 9 , Article number: 24 ( 2023 ) Cite this article
47k Accesses
83 Citations
251 Altmetric
Metrics details
- Paediatric research
The prevalence of child and adolescent obesity has plateaued at high levels in most high-income countries and is increasing in many low-income and middle-income countries. Obesity arises when a mix of genetic and epigenetic factors, behavioural risk patterns and broader environmental and sociocultural influences affect the two body weight regulation systems: energy homeostasis, including leptin and gastrointestinal tract signals, operating predominantly at an unconscious level, and cognitive–emotional control that is regulated by higher brain centres, operating at a conscious level. Health-related quality of life is reduced in those with obesity. Comorbidities of obesity, including type 2 diabetes mellitus, fatty liver disease and depression, are more likely in adolescents and in those with severe obesity. Treatment incorporates a respectful, stigma-free and family-based approach involving multiple components, and addresses dietary, physical activity, sedentary and sleep behaviours. In adolescents in particular, adjunctive therapies can be valuable, such as more intensive dietary therapies, pharmacotherapy and bariatric surgery. Prevention of obesity requires a whole-system approach and joined-up policy initiatives across government departments. Development and implementation of interventions to prevent paediatric obesity in children should focus on interventions that are feasible, effective and likely to reduce gaps in health inequalities.
Similar content being viewed by others

Management for children and adolescents with overweight and obesity: a recommendations mapping

Unraveling Complexity about Childhood Obesity and Nutritional Interventions: Modeling Interactions Among Psychological Factors

Pediatric weight management interventions improve prevalence of overeating behaviors
Introduction.
The prevalence of child and adolescent obesity remains high and continues to rise in low-income and middle-income countries (LMICs) at a time when these regions are also contending with under-nutrition in its various forms 1 , 2 . In addition, during the COVID-19 pandemic, children and adolescents with obesity have been more likely to have severe COVID-19 requiring hospitalization and mechanical ventilation 3 . At the same time, the pandemic was associated with rising levels of childhood obesity in many countries. These developments are concerning, considering that recognition is also growing that paediatric obesity is associated with a range of immediate and long-term negative health outcomes, a decreased quality of life 4 , 5 , an increased presentation to health services 6 and increased economic costs to individuals and society 7 .
Body weight is regulated by a range of energy homeostatic and cognitive–emotional processes and a multifactorial interplay of complex regulatory circuits 8 . Paediatric obesity arises when multiple environmental factors — covering preconception and prenatal exposures, as well as broader changes in the food and physical activity environments — disturb these regulatory processes; these influences are now widespread in most countries 9 .
The treatment of obesity includes management of obesity-associated complications, a developmentally sensitive approach, family engagement, and support for long-term behaviour changes in diet, physical activity, sedentary behaviours and sleep 10 . New evidence highlights the role, in adolescents with more severe obesity, of bariatric surgery 11 and pharmacotherapy, particularly the potential for glucagon-like peptide 1 (GLP1) receptor agonists 12 .
Obesity prevention requires a whole-system approach, with policies across all government and community sectors systematically taking health into account, avoiding harmful health impacts and decreasing inequity. Programmatic prevention interventions operating ‘downstream’ at the level of the child and family, as well as ‘upstream’ interventions at the level of the community and broader society, are required if a step change in tackling childhood obesity is to be realized 13 , 14 .
In this Primer, we provide an overview of the epidemiology, causes, pathophysiology and consequences of child and adolescent obesity. We discuss diagnostic considerations, as well as approaches to its prevention and management. Furthermore, we summarize effects of paediatric obesity on quality of life, and open research questions.
Epidemiology
Definition and prevalence.
The World Health Organization (WHO) defines obesity as “abnormal or excessive fat accumulation that presents a risk to health” 15 . Paediatric obesity is defined epidemiologically using BMI, which is adjusted for age and sex because of the physiological changes in BMI during growth 16 . Global prevalence of paediatric obesity has risen markedly over the past four decades, initially in high-income countries (HICs), but now also in many LMICs 1 .
Despite attempts to standardize the epidemiological classification, several definitions of paediatric obesity are in use; hence, care is needed when comparing prevalence rates. The 2006 WHO Child Growth Standard, for children aged 0 to 5 years, is based on longitudinal observations of multiethnic populations of children with optimal infant feeding and child-rearing conditions 17 . The 2007 WHO Growth Reference is used for the age group 5–19 years 18 , and the 2000 US Centers for Disease Control and Prevention (CDC) Growth Charts for the age group 2–20 years 19 . The WHO and CDC definitions based on BMI-for-age charts are widely used, including in clinical practice. By contrast, the International Obesity Task Force (IOTF) definition, developed from nationally representative BMI data for the age group 2–18 years from six countries, is used exclusively for epidemiological studies 20 .
For the age group 5–19 years, between 1975 and 2016, the global prevalence of obesity (BMI >2 standard deviations (SD) above the median of the WHO growth reference) increased around eightfold to 5.6% in girls and 7.8% in boys 1 . Rates have plateaued at high levels in many HICs but have accelerated in other regions, particularly in parts of Asia. For the age group 2–4 years, between 1980 and 2015, obesity prevalence (IOTF definition, equivalent to an adult BMI of ≥30 kg/m 2 ) increased from 3.9% to 7.2% in boys and from 3.7% to 6.4% in girls 21 . Obesity prevalence is highest in Polynesia and Micronesia, the Middle East and North Africa, the Caribbean and the USA (Fig. 1 ). Variations in prevalence probably reflect different background levels of obesogenic environments, or the sum total of the physical, economic, policy, social and cultural factors that promote obesity 22 . Obesogenic environments include those with decreased active transport options, a ubiquity of food marketing directed towards children, and reduced costs and increased availability of nutrient-poor, energy-dense foods. Particularly in LMICs, the growth of urbanization, new forms of technology and global trade have led to reduced physical activity at work and leisure, a shift towards Western diets, and the expansion of transnational food and beverage companies to shape local food systems 23 .
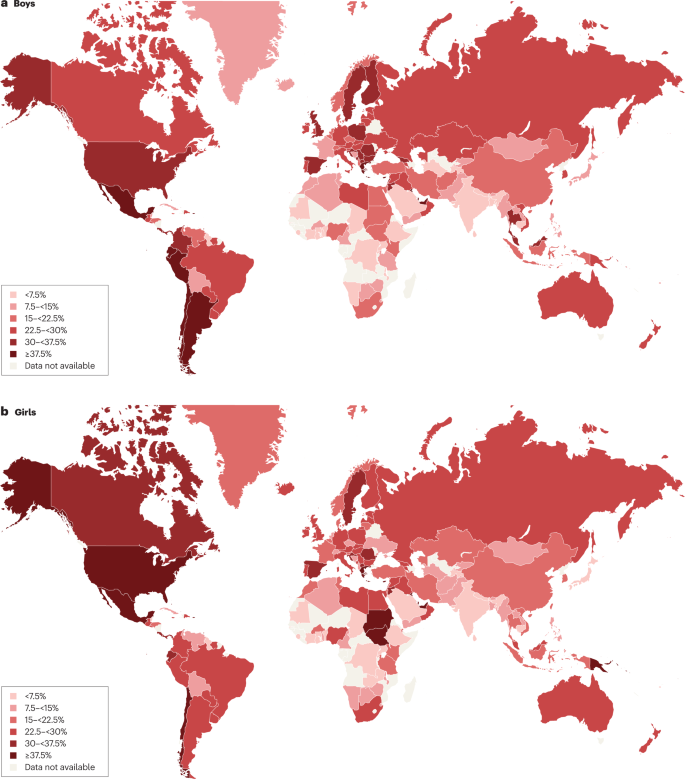
Maps showing the proportions of children and adolescents living with overweight or obesity (part a , boys; part b , girls) according to latest available data from the Global Obesity Observatory . Data might not be comparable between countries owing to differences in survey methodology.
The reasons for varying sex differences in prevalence in different countries are unclear but may relate to cultural variations in parental feeding practices for boys and girls and societal ideals of body size 24 . In 2016, obesity in the age group 5–19 years was more prevalent in girls than in boys in sub-Saharan Africa, Oceania and some middle-income countries in other regions, whereas it was more prevalent in boys than in girls in all HICs, and in East and South-East Asia 21 . Ethnic and racial differences in obesity prevalence within countries are often assumed to mirror variations in social deprivation and other social determinants of obesity. However, an independent effect of ethnicity even after adjustment for socioeconomic status has been documented in the UK, with Black and Asian boys in primary school having higher prevalence of obesity than white boys 25 .
Among individuals with obesity, very high BMI values have become more common in the past 15 years. The prevalence of severe obesity (BMI ≥120% of the 95th percentile (CDC definition), or ≥35 kg/m 2 at any age 26 , 27 ) has increased in many HICs, accounting for one-quarter to one-third of those with obesity 28 , 29 . Future health risks of paediatric obesity in adulthood are well documented. For example, in a data linkage prospective study in Israel with 2.3 million participants who had BMI measured at age 17 years, those with obesity (≥95th percentile BMI for age) had a much higher risk of death from coronary heart disease (HR 4.9, 95% CI 3.9–6.1), stroke (HR 2.6, 95% CI 1.7–4.1) and sudden death (HR 2.1, 95% CI 1.5–2.9) compared with those whose BMI fell between the 5th and 24th percentiles 30 .
Causes and risk factors
Early life is a critical period for childhood obesity development 9 , 31 , 32 , 33 . According to the Developmental Origins of Health and Disease framework, the early life environment may affect organ structure and function and influence health in later life 34 , 35 . Meta-analyses have shown that preconception and prenatal environmental exposures, including high maternal pre-pregnancy BMI and, to a lesser extent, gestational weight gain, as well as gestational diabetes and maternal smoking, are associated with childhood obesity, potentially through effects on the in utero environment 33 , 36 , 37 , 38 . Paternal obesity is also associated with childhood obesity 33 . Birthweight, reflecting fetal growth, is a proxy for in utero exposures. Both low and high birthweights are associated with later adiposity, with high birthweight linked to increased BMI and low birthweight to central obesity 33 , 39 .
Growth trajectories in early life are important determinants of later adiposity. Rapid weight gain in early childhood is associated with obesity in adolescence 32 . Also, later age and higher BMI at adiposity peak (the usual peak in BMI around 9 months of age), as well as earlier age at adiposity rebound (the lowest BMI reached between 4 and 7 years of age), are associated with increased adolescent and adult BMI 40 , 41 . Specific early life nutritional factors, including a lower protein content in formula food, are consistently associated with a lower risk of childhood obesity 42 , 43 . These also include longer breastfeeding duration, which is generally associated with a lower risk of childhood obesity 42 . However, some controversy exists, as these effects are affected by multiple sociodemographic confounding factors and their underlying mechanisms remain uncertain 44 . Some studies comparing higher and lower infant formula protein content have reported that the higher protein group have a greater risk of subsequent obesity, especially in early childhood 41 , 42 ; however, one study with a follow-up period until age 11 years found no significant difference in the risk of obesity, but an increased risk of overweight in the high protein group was still observed 42 , 43 , 45 . A high intake of sugar-sweetened beverages is associated with childhood obesity 33 , 46 .
Many other behavioural factors are associated with an increased risk of childhood obesity, including increased screen time, short sleep duration and poor sleep quality 33 , 47 , reductions in physical activity 48 and increased intake of energy-dense micronutrient-poor foods 49 . These have been influenced by multiple changes in the past few decades in the broader social, economic, political and physical environments, including the widespread marketing of food and beverages to children, the loss of walkable green spaces in many urban environments, the rise in motorized transport, rapid changes in the use of technology, and the move away from traditional foods to ultraprocessed foods.
Obesity prevalence is inextricably linked to relative social inequality, with data suggesting a shift in prevalence over time towards those living with socioeconomic disadvantage, and thus contributes to social inequalities. In HICs, being in lower social strata is associated with a higher risk of obesity, even in infants and young children 50 , whereas the opposite relationship occurs in middle-income countries 51 . In low-income countries, the relationship is variable, and the obesity burden seems to be across socioeconomic groups 52 , 53 .
Overall, many environmental, lifestyle, behavioural and social factors in early life are associated with childhood obesity. These factors cannot be seen in isolation but are part of a complex interplay of exposures that jointly contribute to increased obesity risk. In addition to multiple prenatal and postnatal environmental factors, genetic variants also have a role in the development of childhood obesity (see section Mechanisms/pathophysiology).
Comorbidities and complications
Childhood obesity is associated with a wide range of short-term comorbidities (Fig. 2 ). In addition, childhood obesity tracks into adolescence and adulthood and is associated with complications across the life course 32 , 41 , 54 , 55 .
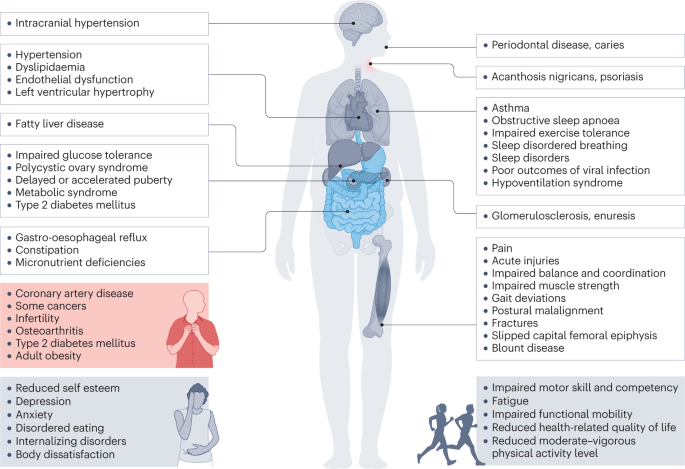
Obesity in children and adolescents can be accompanied by various other pathologies. In addition, childhood obesity is associated with complications and disorders that manifest in adulthood (red box).
Increased BMI, especially in adolescence, is linked to a higher risk of many health outcomes, including metabolic disorders, such as raised fasting glucose, impaired glucose tolerance, type 2 diabetes mellitus (T2DM), metabolic syndrome and fatty liver disease 56 , 57 , 58 , 59 . Other well-recognized obesity-associated complications include coronary heart disease, asthma, obstructive sleep apnoea syndrome (itself associated with metabolic dysfunction and inflammation) 60 , orthopaedic complications and a range of mental health outcomes including depression and low self-esteem 27 , 55 , 57 , 61 , 62 , 63 .
A 2019 systematic review showed that children and adolescents with obesity are 1.4 times more likely to have prediabetes, 1.7 times more likely to have asthma, 4.4 times more likely to have high blood pressure and 26.1 times more likely to have fatty liver disease than those with a healthy weight 64 . In 2016, it was estimated that, at a global level by 2025, childhood obesity would lead to 12 million children aged 5–17 years with glucose intolerance, 4 million with T2DM, 27 million with hypertension and 38 million with fatty liver disease 65 . These high prevalence rates have implications for both paediatric and adult health services.
Mechanisms/pathophysiology
Body weight regulation.
Body weight is regulated within narrow limits by homeostatic and cognitive–emotional processes and a multifactorial interplay of hormones and messenger substances in complex regulatory circuits (Fig. 3 ). When these regulatory circuits are disturbed, an imbalance between energy intake and expenditure leads to obesity or to poor weight gain. As weight loss is much harder to achieve than weight gain in the long term due to the regulation circuits discussed below, the development of obesity is encouraged by modern living conditions, which enable underlying predispositions for obesity to become manifest 8 , 66 .
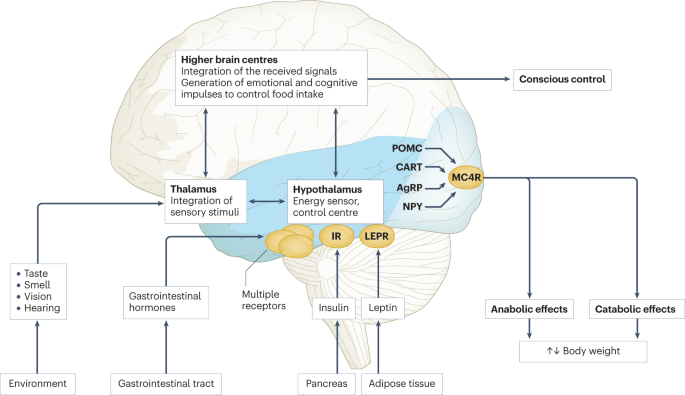
Body weight is predominantly regulated by two systems: energy homeostasis and cognitive–emotional control. Both homeostatic and non-homeostatic signals are processed in the brain, involving multiple hormone and receptor cascades 217 , 218 , 219 . This overview depicts the best-known regulatory pathways. The homeostatic system, which is mainly regulated by brain centres in the hypothalamus and brainstem, operates on an unconscious level. Both long-term signals from the energy store in adipose tissue (for example, leptin) and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status. During gastric distension or after the release of gastrointestinal hormones (multiple receptors are involved) and insulin, a temporary feeling of fullness is induced. The non-homeostatic or hedonic system is regulated by higher-level brain centres and operates at the conscious level. After integration in the thalamus, homeostatic signals are combined with stimuli from the environment, experiences and emotions; emotional and cognitive impulses are then induced to control food intake. Regulation of energy homeostasis in the hypothalamus involves two neuron types of the arcuate nucleus: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). Leptin stimulates these neurons via specific leptin receptors (LEPR) inducing anabolic effects in case of decreasing leptin levels and catabolic effects in case of increasing leptin levels. Leptin inhibits the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production resulting in the feeling of hunger. Leptin directly stimulates POMC production in POMC neurons. POMC is cleaved into different hormone polypeptides including α-melanocyte-stimulating hormone which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. CART, cocaine and amphetamine responsive transcript; IR, insulin receptor.
In principle, there are two main systems in the brain which regulate body weight 8 , 66 (Fig. 3 ): energy homeostasis and cognitive–emotional control. Energy homeostasis is predominantly regulated by brain centres in the hypothalamus and brainstem and operates at an unconscious level. Both long-term signals from the adipose tissue energy stores and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status 8 , 66 . For example, negative energy balance leading to reduced fat mass results in reduced leptin levels, a permanently reduced urge to exercise and an increased feeling of hunger. During gastric distension or after the release of gastrointestinal hormones and insulin, a temporary feeling of fullness is induced 8 , 66 . Cognitive–emotional control is regulated by higher brain centres and operates at a conscious level. Here, the homeostatic signals are combined with stimuli from the environment (sight, smell and taste of food), experiences and emotions 8 , 66 . Disorders at the level of cognitive–emotional control mechanisms include emotional eating as well as eating disorders. For example, the reward areas in the brain of people with overweight are more strongly activated by high-calorie foods than those in the brain of people with normal weight 67 . Both systems interact with each other, and the cognitive–emotional system is strongly influenced by the homeostatic control circuits.
Disturbances in the regulatory circuits of energy homeostasis can be genetically determined, can result from disease or injury to the regulatory centres involved, or can be caused by prenatal programming 8 , 66 . If the target value of body weight has been shifted, the organism tries by all means (hunger, drive) to reach the desired higher weight. These disturbed signals of the homeostatic system can have an imperative, irresistible character, so that a conscious influence on food intake is no longer effectively possible 8 , 66 . The most important disturbances of energy homeostasis are listed in Table 1 .
The leptin pathway
The peptide hormone leptin is primarily produced by fat cells. Its production depends on the amount of adipose tissue and the energy balance. A negative energy balance during fasting results in a reduction of circulating leptin levels by 50% after 24 h (ref. 68 ). In a state of weight loss, leptin production is reduced 69 . In the brain, leptin stimulates two neuron types of the arcuate nucleus in the hypothalamus via specific leptin receptors: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). High leptin levels inhibit the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production. By contrast, leptin directly stimulates POMC production in POMC neurons (Fig. 3 ). POMC is a hormone precursor that is cleaved into different hormone polypeptides by specific enzymes, such as prohormone convertase 1 (PCSK1). This releases α-melanocyte-stimulating hormone (α-MSH) which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. Rare, functionally relevant mutations in the genes for leptin and leptin receptor, POMC , PCSK1/3 or MC4R lead to extreme obesity in early childhood. These forms of obesity are potential indications for specific pharmacological treatments, for example setmelanotide 70 , 71 . MC4R mutations are the most common cause of monogenic obesity, as heterozygous mutations can be symptomatic depending on the functional impairment and with variable penetrance and expression. Other genes have been identified, in which rare heterozygous pathological variants are also associated with early onset obesity (Table 1 ).
Pathological changes in adipose tissue
Adipose tissue can be classified into two types, white and brown adipose tissue. White adipose tissue comprises unilocular fat cells and brown adipose tissue contains multilocular fat cells, which are rich in mitochondria 72 . A third type of adipocyte, beige adipocytes, within the white adipose tissue are induced by prolonged exposure to cold or adrenergic signalling, and show a brown adipocyte-like morphology 72 . White adipose tissue has a large potential to change its volume to store energy and meet the metabolic demands of the body. The storage capacity and metabolic function of adipose tissue depend on the anatomical location of the adipose tissue depot. Predominant enlargement of white adipose tissue in the visceral, intra-abdominal area (central obesity) is associated with insulin resistance and an increased risk of metabolic disease development before puberty. Accumulation of adipose tissue in the hips and flanks has no adverse effect and may be protective against metabolic syndrome. In those with obesity, adipose tissue is characterized by an increased number of adipocytes (hyperplasia), which originate from tissue-resident mesenchymal stem cells, and by enlarged adipocytes (hypertrophy) 73 . Adipocytes with a very large diameter reach the limit of the maximal oxygen diffusion distance, resulting in hypoxia, the development of an inflammatory expression profile (characterized by, for example, leptin, TNF and IL-6) and adipocyte necrosis, triggering the recruitment of leukocytes. Resident macrophages switch from the anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype, which is associated with insulin resistance, further promoting local sterile inflammation and the development of fibrotic adipose tissue. This process limits the expandability of the adipose tissue for further storage of triglycerides. In the patient, the increase in fat mass in obesity is associated with insulin resistance and systemic low-grade inflammation characterized by elevated serum levels of C-reactive protein and pro-inflammatory cytokines. The limitation of adipose tissue expandability results in storage of triglycerides in other organs, such as the liver, muscle and pancreas 74 .
Genetics and epigenetics in the general population
Twin studies have found heritability estimates for BMI of up to 70% 75 , 76 . In contrast to rare monogenic forms of obesity, which are often caused by a single genetic defect with a large effect, the genetic background of childhood obesity in the general population is shaped by the joint effects of many common genetic variants, each of which individually makes a small contribution to the phenotype. For adult BMI, genome-wide association studies, which examine associations of millions of such variants across the genome at the same time, have identified around 1,000 genetic loci 77 . The largest genome-wide association studies in children, which include much smaller sample sizes of up to 60,000 children, have identified 25 genetic loci for childhood BMI and 18 for childhood obesity, the majority of which overlap 78 , 79 . There is also a clear overlap with genetic loci identified in adults, for example for FTO , MC4R and TMEM18 , but this overlap is not complete, some loci are specific to early life BMI, or have a relatively larger contribution in childhood 78 , 79 , 80 . These findings suggest that biological mechanisms underlying obesity in childhood are mostly similar to those in adulthood, but the relative influence of these mechanisms may differ at different phases of life.
The role of epigenetic processes in childhood and adolescent obesity has gained increasing attention. In children, several studies found associations between DNA methylation and BMI 81 , 82 , 83 , 84 , but a meta-analysis including data from >4,000 children identified only minimal associations 85 . Most studies support the hypothesis that DNA methylation changes are predominantly a consequence rather than a cause of obesity, which may explain the lower number of identified (up to 12) associations in children, in whom duration of exposure to a higher BMI is shorter than in adults, in whom associations with DNA methylation at hundreds of sites have been identified 85 , 86 , 87 . In addition to DNA methylation, some specific circulating microRNAs have been found to be associated with obesity in childhood 84 .
The field of epigenetic studies in childhood obesity is relatively young and evolving quickly. Future studies will need to focus on defining robust associations in blood as well as other tissues and on identifying cause-and-effect relationships. In addition, other omics, such as metabolomics and proteomics, are promising areas that may contribute to an improved aetiological understanding or may provide biological signatures that can be used as predictive or prognostic markers of childhood obesity and its comorbidities.
Parental obesity and childhood obesity
There is an established link between increased parental BMI and increased childhood BMI 88 , 89 . This link may be due to shared genetics, shared environment, a direct intrauterine effect of maternal BMI or a combination of these factors. In the case of shared genetics, the child inherits BMI-increasing genetic variants from one or both parents. Shared environmental factors, such as diet or lifestyle, may also contribute to an increased BMI in both parents and child. In addition, maternal obesity might create an intrauterine environment that programmes metabolic processes in the fetus, which increases the risk of childhood obesity. Some studies show larger effects of maternal than paternal BMI, indicating a potential causal intrauterine mechanism of maternal obesity, but evidence showing similar maternal and paternal effects is increasing. The data may indicate that there is only a limited direct intrauterine effect of maternal obesity on childhood obesity; rather, genetic effects inherited from the mother or father, or both, and/or shared environmental factors may contribute to childhood obesity risk 90 , 91 , 92 , 93 , 94 , 95 .
Diagnosis, screening and prevention
Diagnostic work-up.
The extent of overweight in clinical practice is estimated using BMI based on national charts 96 , 97 , 98 , 99 , 100 . Of note, the clinical classification of overweight or obesity differ depending on the BMI charts used and national recommendations; hence, local guidelines should be referred to. For example, the US CDC Growth Charts and several others use the 85th and 95th centile cut-points to denote overweight and obesity, respectively 19 . The WHO Growth Reference for children aged 5–19 years defines cut-points for overweight and obesity as a BMI-for-age greater than +1 and +2 SDs for BMI for age, respectively 18 . For children <5 years of age, overweight and obesity are defined as weight-for-height greater than +2 and +3 SDs, respectively, above the WHO Child Growth Standards median 17 . The IOTF and many countries in Europe use cut-points of 85th, 90th and 97th to define overweight, obesity and extreme obesity 26 .
BMI as an indirect measurement of body fat has some limitations; for example, pronounced muscle tissue leads to an increase in BMI, and BMI is not independent of height. In addition, people of different ethnicities may have different cut-points for obesity risk; for example, cardiometabolic risk occurs at lower BMI values in individuals with south Asian than in those with European ancestry 101 . Thus, BMI is best seen as a convenient screening tool that is supplemented by clinical assessment and investigations.
Other measures of body fat may help differentiate between fat mass and other tissues. Some of these tools are prone to low reliability, such as body impedance analyses (high day-to-day variation and dependent on level of fluid consumption) or skinfold thickness (high inter-observer variation), or are more expensive or invasive, such as MRI, CT or dual-energy X-ray absorptiometry, than simpler measures of body composition or BMI assessment.
Primary diseases rarely cause obesity in children and adolescents (<2%) 102 . However, treatable diseases should be excluded in those with obesity. A suggested diagnostic work-up is summarized in Fig. 4 . Routine measurement of thyroid-stimulating hormone (TSH) is not recommended 96 . Moderately elevated TSH levels (usually <10 IU/l) are frequently observed in obesity and are a consequence, and not a cause, of obesity 103 . In a growing child with normal height velocity, a normal BMI at the age of 2 years and normal cognitive development, no further diagnostic steps are necessary to exclude primary diseases 96 , 104 .
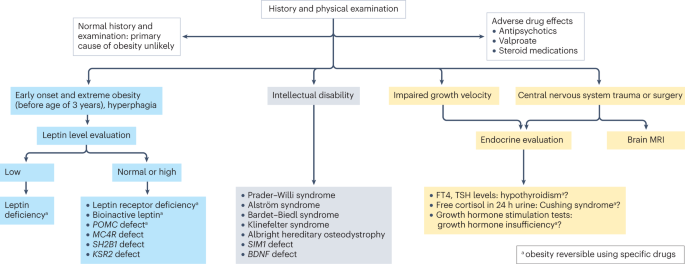
Concerning findings from a detailed medical history and physical examination will lead to further examinations. In individuals with early onset, extreme obesity (before age 3 years) and signs of hyperphagia, serum leptin level should be measured to rule out the extremely rare condition of congenital leptin deficiency. In individuals with normal or high leptin levels, genetic testing is indicated to search for monogenetic obesity. In individuals with intellectual disability, a syndromic disease may be present. Signs of impaired growth velocity or the history of central nervous system trauma or surgery will result in deeper endocrine evaluation and/or brain MRI. BDNF , brain-derived neurotropic factor; FT4, free thyroxin; KSR2 , kinase suppressor of ras 2; MC4R , melanocortin 4 receptor; POMC , pro-opiomelanocortin; SH2B1 , Src-homology 2 (SH2) B adapter protein 1; SIM1 , single-minded homologue 1; TSH, thyroid-stimulating hormone.
Clinical findings which need no further examination include pseudogynaecomastia (adipose tissue mimicking breast development; differentiated from breast tissue by ultrasonography), striae (caused by rapid weight increase) and a hidden penis in suprapubic adipose tissue (differentiated from micropenis by measurement of stretched penis length while pressing down on the suprapubic adipose tissue) 96 , 105 . Girls with obesity tend to have an earlier puberty onset (usually at around 8–9 years of age) and boys with severe obesity may have a delayed puberty onset (usually at around 13–14 years of age) 106 . Thus, if pubertal onset is slightly premature in girls or slightly delayed in boys, no further endocrine assessment is necessary.
Assessment of obesity-associated comorbidities
A waist to height ratio of >0.5 is a simple tool to identify central obesity 107 , 108 . Screening for cardiometabolic risk factors and fatty liver disease is recommended, especially in adolescents, and in those with more severe obesity or central adiposity, a strong family history of T2DM or premature heart disease, or relevant clinical symptoms, such as high blood pressure or acanthosis nigricans 96 , 97 , 98 , 99 , 109 . Investigations generally include fasting glucose levels, lipid profile, liver function and glycated haemoglobin, and might include an oral glucose tolerance test, polysomnography, and additional endocrine tests for polycystic ovary syndrome 96 , 97 , 98 , 99 .
T2DM in children and adolescents often occurs in the presence of a strong family history and may not be related to obesity severity 110 . T2DM onset usually occurs during puberty, a physiological state associated with increased insulin resistance 111 and, therefore, screening for T2DM should be considered in children and adolescents with obesity and at least one risk factor (family history of T2DM or features of metabolic syndrome) starting at pubertal onset 112 . As maturity-onset diabetes of the young (MODY) type II and type III are more frequent than T2DM in children and adolescents in many ethnicities, genetic screening for MODY may be appropriate 112 . Furthermore, type 1 diabetes mellitus (T1DM) should be excluded by measurement of autoantibodies in any individual with suspected diabetes with obesity. The differentiation of T2DM from MODY and T1DM is important as the diabetes treatment approaches differ 112 .
Several comorbidities of obesity should be considered if specific symptoms occur 96 , 109 . For polycystic ovary syndrome in hirsute adolescent girls with oligomenorrhoea or amenorrhoea, moderately increased testosterone levels and decreased sex hormone binding globulin levels are typical laboratory findings 113 . Obstructive sleep apnoea can occur in those with more severe obesity and who snore, have daytime somnolence or witnessed apnoeas. Diagnosis is made by polysomnography 114 . Minor orthopaedic disorders, such as flat feet and genu valgum, are frequent in children and adolescents with obesity and may cause pain. Major orthopaedic complications include slipped capital femoral epiphyses (acute and chronic), which manifest with hip and knee pain in young adolescents and are characterized by reduced range of hip rotation and waddling gait; and Blount disease (tibia vara), typically occurring in children aged 2–5 years 105 , 115 . In addition, children and adolescents with extreme obesity frequently have increased dyspnoea and decreased exercise capacity. A heightened demand for ventilation, elevated work of breathing, respiratory muscle inefficiency and diminished respiratory compliance are caused by increased truncal fat mass. This may result in a decreased functional residual capacity and expiratory reserve volume, ventilation to perfusion ratio abnormalities and hypoxaemia, especially when supine. However, conventional respiratory function tests are only mildly affected by obesity except in extreme cases 116 . Furthermore, gallstones should be suspected in the context of abdominal pain after rapid weight loss, which can be readily diagnosed via abdominal ultrasonography 105 . Finally, pseudotumor cerebri may present with chronic headache, and depression may present with flat affect, chronic fatigue and sleep problems 105 .
Obesity in adolescents can also be associated with disordered eating, eating disorders and other psychological disorders 117 , 118 . If suspected, assessment by a mental health professional is recommended.
A comprehensive approach
The 2016 report of the WHO Commission on Ending Childhood Obesity stated that progress in tackling childhood obesity has been slow and inconsistent, with obesity prevention requiring a whole-of-government approach in which policies across all sectors systematically take health into account, avoiding harmful health impacts and, therefore, improving population health and health equity 13 , 119 . The focus in developing and implementing interventions to prevent obesity in children should be on interventions that are feasible, effective and likely to reduce health inequalities 14 . Importantly, the voices of children and adolescents living with social disadvantage and those from minority groups must be heard if such interventions are to be effective and reduce inequalities 120 .
Figure 5 presents a system for the prevention of childhood obesity within different domains of the socioecological model 121 and highlights opportunities for interventions. These domains can be described on a continuum, from (most downstream) individual and interpersonal (including parents, peers and wider family) through to organizational (including health care and schools), community (including food, activity and environment), society (including media and finally cultural norms) and (most upstream) public policy (from local to national level). Interventions to prevent childhood obesity can be classified on the Nuffield intervention ladder 122 . This framework was proposed by the Nuffield Council on Bioethics in 2007 (ref. 122 ) and distributes interventions on the ladder steps depending on the degree of agency required by the individual to make the behavioural changes that are the aim of the intervention. The bottom step of the ladder includes interventions that provide information, which requires the highest agency and relies on a child, adolescent and/or family choosing (and their ability to choose) to act on that information and change behaviour. The next steps of the ladder are interventions that enable choice, guide choice through changing the default policy, guide choice through incentives, guide choice through disincentives, or restrict choice. On the top-most step of the ladder (lowest agency required) are interventions that eliminate choice.
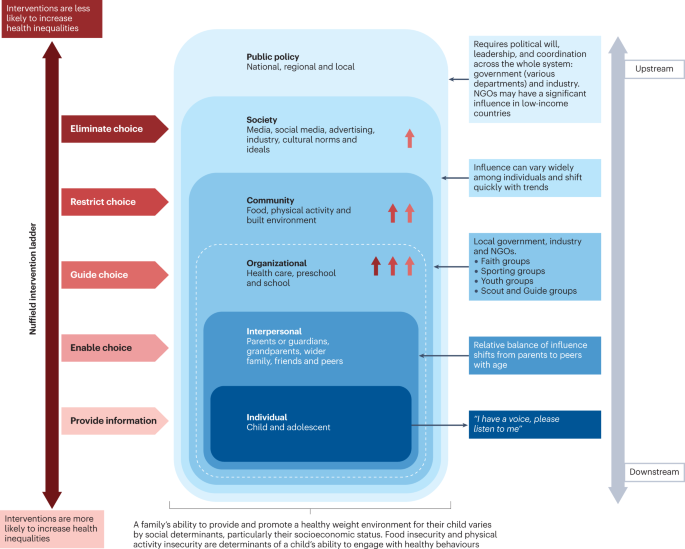
This schematic integrates interventions that were included in a Cochrane review 127 of 153 randomized controlled trials of interventions to prevent obesity in children and are high on the Nuffield intervention ladder 122 . The Nuffield intervention ladder distributes interventions depending on the degree of agency required for the behavioural changes that are the aim of the intervention. The socioecological model 121 comprises different domains (or levels) from the individual up to public policy. Interventions targeting the individual and interpersonal domains can be described as downstream interventions, and interventions within public policy can be described as the highest level of upstream interventions. Within each of these domains, arrow symbols with colours corresponding to the Nuffield intervention ladder category are used to show interventions that were both included in the Cochrane review 127 and that guide, restrict or eliminate choice as defined by the Nuffield intervention ladder 122 . Upstream interventions, and interventions on the top steps of the Nuffield ladder, are more likely to reduce inequalities. NGO, non-governmental organization.
Downstream and high-agency interventions (on the bottom steps of the Nuffield ladder) are more likely to result in intervention-generated inequalities 123 . This has been elegantly described and evidenced, with examples from the obesity prevention literature 124 , 125 . A particularly strong example is a systematic review of 38 interventions to promote healthy eating that showed that food price (an upstream and low-agency intervention) seemed to decrease inequalities, all interventions that combined taxes and subsidies consistently decreased inequalities, and downstream high-agency interventions, especially dietary counselling, seemed to increase inequalities 126 .
Effectiveness of prevention interventions
A 2019 Cochrane review of interventions to prevent obesity in children 127 included 153 randomized controlled trials (RCTs), mainly in HICs (12% were from middle-income countries). Of these RCTs, 56% tested interventions in children aged 6–12 years, 24% in children aged 0–5 years, and 20% in adolescents aged 13–18 years. The review showed that diet-only interventions to prevent obesity in children were generally ineffective across all ages. Interventions combining diet and physical activity resulted in modest benefits in children aged 0–12 years but not in adolescents. However, physical activity-only interventions to prevent obesity were effective in school-age children (aged 5–18 years). Whether the interventions were likely to work equitably in all children was investigated in 13 RCTs. These RCTs did not indicate that the strategies increased inequalities, although most of the 13 RCTs included relatively homogeneous groups of children from disadvantaged backgrounds.
The potential for negative unintended consequences of obesity prevention interventions has received much attention 128 . The Cochrane review 127 investigated whether children were harmed by any of the strategies; for example, by having injuries, losing too much weight or developing damaging views about themselves and their weight. Of the few RCTs that did monitor these outcomes, none found any harms in participants.
Intervention levels
Most interventions (58%) of RCTs in the Cochrane review aimed to change individual lifestyle factors via education-based approaches (that is, simply provide information) 129 . In relation to the socioecological model, only 11 RCTs were set in the food and physical activity environment domain, and child care, preschools and schools were the most common targets for interventions. Of note, no RCTs were conducted in a faith-based setting 130 . Table 2 highlights examples of upstream interventions that involve more than simply providing information and their classification on the Nuffield intervention ladder.
Different settings for interventions to prevent childhood obesity, including preschools and schools, primary health care, community settings and national policy, offer different opportunities for reach and effectiveness, and a reduction in inequalities.
Preschools and schools are key settings for public policy interventions for childhood obesity prevention, and mandatory and voluntary food standards and guidance on physical education are in place in many countries. Individual schools are tasked with translating and implementing these standards and guidance for their local context. Successful implementation of a whole-school approach, such as that used in the WHO Nutrition-Friendly Schools Initiative 131 , is a key factor in the effectiveness of interventions. Careful consideration should be given to how school culture can, and needs to, be shifted by working with schools to tailor the approach and manage possible staff capacity issues, and by building relationships within and outside the school gates to enhance sustainability 132 , 133 .
Primary health care offers opportunities for guidance for obesity prevention, especially from early childhood to puberty. Parent-targeted interventions conducted by clinicians in health-care or community settings have the strongest level of evidence for their effectiveness in reducing BMI z -score at age 2 years 134 . These interventions include group programmes, clinic nurse consultations, mobile phone text support or nurse home visiting, and focusing on healthy infant feeding, healthy childhood feeding behaviours and screen time.
A prospective individual participant data meta-analysis of four RCTs involving 2,196 mother–baby dyads, and involving nurse home visiting or group programmes, resulted in a small but significant reduction in BMI in infants in the intervention groups compared with control infants at age 18–24 months 134 . Improvements were also seen in television viewing time, breastfeeding duration and feeding practices. Interventions were more effective in settings with limited provision of maternal and child health services in the community. However, effectiveness diminished by age 5 years without further intervention, highlighting the need for ongoing interventions at each life stage 135 . Evidence exists that short-duration interventions targeting sleep in very early childhood may be more effective than nutrition-targeted interventions in influencing child BMI at age 5 years 136 .
Primary care clinicians can provide anticipatory guidance, as a form of primary prevention, to older children, adolescents and their families, aiming to support healthy weight and weight-related behaviours. Clinical guidelines recommend that clinicians monitor growth regularly, and provide guidance on healthy eating patterns, physical activity, sedentary behaviours and sleep patterns 97 , 100 . Very few paediatric trials have investigated whether this opportunistic screening and advice is effective in obesity prevention 100 . A 2021 review of registered RCTs for the prevention of obesity in infancy found 29 trials 137 , of which most were delivered, or were planned to be delivered, in community health-care settings, such as nurse-led clinics. At the time of publication, 11 trials had reported child weight-related outcomes, two of which showed a small but significant beneficial effect on BMI at age 2 years, and one found significant improvements in the prevalence of obesity but not BMI. Many of the trials showed improvements in practices, such as breastfeeding and screen time.
At the community level, local public policy should be mindful of the geography of the area (such as urban or rural) and population demographics. Adolescents usually have more freedom in food and beverage choices made outside the home than younger children. In addition, physical activity levels usually decline and sedentary behaviours rise during adolescence, particularly in girls 138 , 139 . These behavioural changes offer both opportunities and barriers for those developing community interventions. On a national societal level, public policies for interventions to prevent obesity in children include the control of advertising of foods and beverages high in fat, sugar and/or salt in some countries. Industry and the media, including social media, can have a considerable influence on the food and physical activity behaviours of children 13 , 119 .
Public policy may target interventions at all domains from the individual to the societal level. The main focus of interventions in most national public policies relies on the ability of individuals to make the behavioural changes that are the aim of the intervention (high-agency interventions) at the individual level (downstream interventions). An equal focus on low-agency and upstream interventions is required if a step change in tackling childhood obesity is to be realized 140 , 141 .
COVID-19 and obesity
Early indications in several countries show rising levels of childhood obesity, and an increase in inequalities in childhood obesity during the COVID-19 pandemic 142 . The substantial disruptions in nutrition and lifestyle habits of children during and since the pandemic include social isolation and addiction to screens 143 . Under-nutrition is expected to worsen in poor countries, but obesity rates could increase in middle-income countries and HICs, especially among vulnerable groups, widening the gap in health and social inequalities 143 . Public health approaches at national, regional and local levels should include strategies that not only prevent obesity and under-nutrition, but also reduce health inequalities.
In summary, although most trials of obesity prevention have occurred at the level of the individual, the immediate family, school or community, effective prevention of obesity will require greater investment in upstream, low-agency interventions.
Treatment goals
Treatment should be centred on the individual and stigma-free (Box 1 ) and may aim for a reduction in overweight and improvement in associated comorbidities and health behaviours. Clinical considerations when determining a treatment approach should include age, severity of overweight and the presence of associated complications 144 , 145 .
Box 1 Strategies for minimizing weight stigma in health care 220 , 221 , 222
Minimizing weight bias in the education of health-care professionals
Improved education of health professionals:
pay attention to the implicit and explicit communication of social norms
include coverage of the broader determinants of obesity
include discussion of harms caused by social and cultural norms and messages concerning body weight
provide opportunities to practise non-stigmatizing care throughout education
Provide causal information focusing on the genetic and/or socioenvironmental determinants of weight.
Provide empathy-invoking interventions, emphasizing size acceptance, respect and human dignity.
Provide a weight-inclusive approach, by emphasizing that all individuals, regardless of size, have the right to equal health care.
Addressing health facility infrastructure and processes
Provide appropriately sized chairs, blood pressure cuffs, weight scales, beds, toilets, showers and gowns.
Use non-stigmatizing language in signage, descriptions of clinical services and other documentation.
Providing clinical leadership and using appropriate language within health-care settings
Senior clinicians and managers should role-model supportive and non-biased behaviours towards people with obesity and indicate that they do not tolerate weight-based discrimination in any form.
Staff should identify the language that individuals prefer in referring to obesity.
Use person-first language, for example a ‘person with obesity’ rather than ‘an obese person’.
Treatment guidelines
Clinical guidelines advise that first-line management incorporates a family-based multicomponent approach that addresses dietary, physical activity, sedentary and sleep behaviours 97 , 99 , 109 , 146 . This approach is foundational, with adjunctive therapies, especially pharmacotherapy and bariatric surgery, indicated under specific circumstances, usually in adolescents with more severe obesity 144 , 145 . Guideline recommendations vary greatly among countries and are influenced by current evidence, and functionality and resourcing of local health systems. Hence, availability and feasibility of therapies differs internationally. In usual clinical practice, interventions may have poorer outcomes than is observed in original studies or anticipated in evidence-based guidelines 147 because implementation of guidelines is more challenging in resource-constrained environments 148 . In addition, clinical trials are less likely to include patients with specialized needs, such as children from culturally diverse populations, those living with social disadvantage, children with complex health problems, and those with severe obesity 149 , 150 .
Behavioural interventions
There are marked differences in individual responses to behavioural interventions, and overall weight change outcomes are often modest. In children aged 6–11 years, a 2017 Cochrane review 150 found that mean BMI z -scores were reduced in those involved in behaviour-changing interventions compared with those receiving usual care or no treatment by only 0.06 units (37 trials; 4,019 participants; low-quality evidence) at the latest follow-up (median 10 months after the end of active intervention). In adolescents aged 12–17 years, another 2017 Cochrane review 149 found that multicomponent behavioural interventions resulted in a mean reduction in weight of 3.67 kg (20 trials; 1,993 participants) and reduction in BMI of 1.18 kg/m 2 (28 trials; 2,774 participants). These effects were maintained at the 24-month follow-up. A 2012 systematic review found significant improvements in LDL cholesterol triglycerides and blood pressure up to 1 year from baseline following lifestyle interventions in children and adolescents 151 .
Family-based behavioural interventions are recommended in national level clinical practice guidelines 97 , 100 , 146 , 152 . They are an important element of intensive health behaviour and lifestyle treatments (IHBLTs) 109 . Family-based approaches use behavioural techniques, such as goal setting, parental monitoring or modelling, taught in family sessions or in individual sessions separately to children and care givers, depending on the child’s developmental level. The priority is to encourage the whole family to engage in healthier behaviours that result in dietary improvement, greater physical activity, and less sedentariness. This includes making changes to the family food environment and requires parental monitoring.
Family-based interventions differ in philosophy and implementation from those based on family systems theory and therapy 153 . All are intensive interventions that require multiple contact hours (26 or more) with trained specialists delivered over an extended period of time (6–12 months) 10 . Changing family lifestyle habits is challenging and expensive, and the therapeutic expertise is not widely available. Moving interventions to primary care settings, delivered by trained health coaches, and supplemented by remote contact (for example by phone), will improve access and equity 154 .
Very few interventions use single psychological approaches. Most effective IHBLTs are multicomponent and intensive (many sessions), and include face-to-face contact. There has been interest in motivational interviewing as an approach to delivery 155 . As client-centred counselling, this places the young person at the centre of their behaviour change. Fundamental to motivational interviewing is the practitioner partnership that helps the young person and/or parents to explore ambivalence to change, consolidate commitment to change, and develop a plan based on their own insights and expertise. Evidence reviews generally support the view that motivational interviewing reduces BMI. Longer interventions (>4 months), those that assess and report on intervention fidelity, and those that target both diet and physical activity are most effective 155 , 156 .
More intensive dietary interventions
Some individuals benefit from more intensive interventions 98 , 144 , 157 , 158 , which include very low-energy diets, very low-carbohydrate diets and intermittent energy restriction 159 . These interventions usually aim for weight loss and are only recommended for adolescents who have reached their final height. These diets are not recommended for long periods of time due to challenges in achieving nutritional adequacy 158 , 160 , and lack of long-term safety data 158 , 161 . However, intensive dietary interventions may be considered when conventional treatment is unsuccessful, or when adolescents with comorbidities or severe obesity require rapid or substantial weight loss 98 . A 2019 systematic review of very low-energy diets in children and adolescents found a mean reduction in body weight of −5.3 kg (seven studies) at the latest follow‐up, ranging from 5 to 14.5 months from baseline 161 .
Pharmacological treatment
Until the early 2020s the only drug approved in many jurisdictions for the treatment of obesity in adolescents was orlistat, a gastrointestinal lipase inhibitor resulting in reduced uptake of lipids and, thereby, a reduced total energy intake 162 . However, the modest effect on weight in combination with gastrointestinal adverse effects limit its usefulness overall 163 .
A new generation of drugs has been developed for the treatment of both T2DM and obesity. These drugs are based on gastrointestinal peptides with effects both locally and in the central nervous system. GLP1 is an incretin that reduces appetite and slows gastric motility. The GLP1 receptor agonist liraglutide is approved for the treatment of obesity in those aged 12 years and older both in the USA and Europe 164 , 165 . Liraglutide, delivered subcutaneously daily at a higher dose than used for T2DM resulted in a 5% better BMI reduction than placebo after 12 months 166 . A 2022 trial of semaglutide, another GLP1 receptor agonist, delivered subcutaneously weekly in adolescents demonstrated 16% weight loss after 68 weeks of treatment, with modest adverse events and a low drop-out rate 12 . Tirzepatide, an agonist of both GLP1 and glucose-dependent insulinotropic polypeptide (GIP), is approved by the FDA for the treatment of T2DM in adults 167 . Subcutaneous tirzepatide weekly in adults with obesity resulted in ~20% weight loss over 72 weeks 168 . Of note, GIP alone increases appetite, but the complex receptor–agonist interaction results in downregulation of the GIP receptors 169 , illustrating why slightly modified agonists exert different effects. A study of the use of tirzepatide in adolescents with T2DM has been initiated but results are not expected before 2027 (ref. 170 ). No trials of tirzepatide are currently underway in adolescents with obesity but without T2DM.
Hypothalamic obesity is difficult to treat. Setmelanotide is a MC4R agonist that reduces weight and improves quality of life in most people with LEPR and POMC mutations 71 . In trials of setmelanotide, 8 of 10 participants with POMC deficiency and 5 of 11 with LEPR deficiency had weight loss of at least 10% at ~1 year. The mean percentage change in most hunger score from baseline was −27.1% and −43.7% in those with POMC deficiency and leptin receptor deficiency, respectively 71 .
In the near future, effective new drugs with, hopefully, an acceptable safety profile will be available that will change the way we treat and set goals for paediatric obesity treatment 171 .
Bariatric surgery
Bariatric surgery is the most potent treatment for obesity in adolescents with severe obesity. The types of surgery most frequently used are sleeve gastrectomy and gastric bypass, both of which reduce appetite 172 . Mechanisms of action are complex, involving changes in gastrointestinal hormones, neural signalling, bile acid metabolism and gut microbiota 173 . Sleeve gastrectomy is a more straightforward procedure and the need for vitamin supplementation is lower than with gastric bypass. However, long-term weight loss may be greater after gastric bypass surgery 174 .
Prospective long-term studies demonstrate beneficial effects of both sleeve gastrectomy and gastric bypass on weight loss and comorbidities in adolescents with severe obesity 175 , 176 . In a 5-year follow-up period, in 161 participants in the US TEEN-LABS study who underwent gastric bypass, mean BMI declined from 50 to 37 kg/m 2 (ref. 11 ). In a Swedish prospective study in 81 adolescents who underwent gastric bypass, the mean decrease in BMI at 5 years was 13.1 kg/m 2 (baseline BMI 45.5 kg/m 2 ) compared with a BMI increase of 3.1 kg/m 2 in the control group 176 . Both studies showed marked inter-individual variations. Negative adverse effects, including gastrointestinal problems, vitamin deficits and reduction in lean body mass, are similar in adults and adolescents. Most surgical complications following bariatric surgery in the paediatric population are minor, occurring in the early postoperative time frame, but 8% of patients may have major perioperative complications 177 . Up to one-quarter of patients may require subsequent related procedures within 5 years 109 . However, many adolescents with severe obesity also have social and psychological problems, highlighting the need for routine and long-term monitoring 109 , 178 .
Recommendations for bariatric surgery in adolescents differ considerably among countries, with information on long-term outcomes emerging rapidly. In many countries, bariatric surgery is recommended only from Tanner pubertal stage 3–4 and beyond, and only in children with severe obesity and cardiometabolic comorbidities 177 . The 2023 American Academy of Pediatrics clinical practice guidelines recommend that bariatric surgery be considered in adolescents ≥13 years of age with a BMI of ≥35 kg/m 2 or 120% of the 95th percentile for age and sex, whichever is lower, as well as clinically significant disease, such as T2DM, non-alcoholic fatty liver disease, major orthopaedic complications, obstructive sleep apnoea, the presence of cardiometabolic risk, or depressed quality of life 109 . For those with a BMI of ≥40 kg/m 2 or 140% of the 95th percentile for age and sex, bariatric surgery is indicated regardless of the presence of comorbidities. Potential contraindications to surgery include correctable causes of obesity, pregnancy and ongoing substance use disorder. The guidelines comment that further evaluation, undertaken by multidisciplinary centres that offer bariatric surgery for adolescents, should determine the capacity of the patient and family to understand the risks and benefits of surgery and to adhere to the required lifestyle changes before and after surgery.
Long-term weight outcomes
Few paediatric studies have investigated long-term weight maintenance after the initial, more intensive, weight loss phase. A 2018 systematic review of 11 studies in children and adolescents showed that a diverse range of maintenance interventions, including support via face-to-face psychobehavioural therapies, individual physician consultations, or adjunctive therapeutic contact via newsletters, mobile phone text or e-mail, led to stabilization of BMI z -score compared with control participants, who had increases in BMI z -score 179 . Interventions that are web-based or use mobile devices may be particularly useful in young people 180 .
One concern is weight regain which occurs after bariatric surgery in general 181 but may be more prevalent in adolescents 176 . For example, in a Swedish prospective study, after 5 years, 25–30% of participants fulfilled the definitions of low surgical treatment effectiveness, which was associated with poorer metabolic outcomes 176 . As with adults, prevention of weight regain for most at-risk individuals might be possible with the combination of lifestyle support and pharmacological treatment 182 . Further weight maintenance strategies and long-term outcomes are discussed in the 2023 American Academy of Pediatrics clinical practice guidelines 109 . The appropriate role and timing of other therapies for long-term weight loss maintenance, such as anti-obesity medications, more intensive dietary interventions and bariatric surgery, are areas for future research.
In summary, management of obesity in childhood and adolescence requires intensive interventions. Emerging pharmacological therapies demonstrate greater short-term effectiveness than behavioural interventions; however, long-term outcomes at ≥2 years remain an important area for future research.
Quality of life
Weight bias describes the negative attitudes to, beliefs about and behaviour towards people with obesity 183 . It can lead to stigma causing exclusion, and discrimination in work, school and health care, and contributes to the inequities common in people with obesity 184 . Weight bias also affects social engagement and psychological well-being of children.
Children and adolescents with obesity score lower overall on health-related quality of life (HRQoL) 4 , 5 . In measures that assess domains of functioning, most score lower in physical functioning, physical/general health and psychosocial areas, such as appearance, and social acceptance and functioning. HRQoL is lowest in treatment-seeking children and in those with more extreme obesity 185 . Weight loss interventions generally increase HRQoL independent of the extent of weight loss 186 , especially in the domains most affected. However, changes in weight and HRQoL are often not strongly correlated. This may reflect a lag in the physical and/or psychosocial benefit from weight change, or the extent of change that is needed to drive change in a child’s self-perception.
Similar observations apply to the literature on self-esteem. Global self-worth is reduced in children and adolescents with obesity, as is satisfaction with physical appearance, athletic competence and social acceptance 187 . Data from intensive interventions suggest the psychological benefit of weight loss may be as dependent on some feature of the treatment environment or supportive social network as the weight loss itself 188 . This may include the daily company of others with obesity, making new friendships, and experienced improvements in newly prioritized competences.
There is a bidirectional relationship between HRQoL and obesity 189 , something also accepted in the relationship with mood disorder. Obesity increases the risk of depression and vice versa, albeit over a longer period of time and which may only become apparent in adulthood 190 . Obesity also presents an increased risk of anxiety 191 .
Structured and professionally delivered weight management interventions ameliorate mood disorder symptoms 192 and improve self-esteem 193 . Regular and extended support are important components beyond losing weight. Such interventions do not increase the risk of eating disorders 194 . This is despite a recognition that binge eating disorder is present in up to 5% of adolescents with overweight or obesity 195 . They are five times more likely to have binge eating symptoms than those with average weight. Importantly, adolescents who do not have access to professionally delivered weight management may be more likely to engage in self-directed dieting, which is implicated in eating disorder development 196 .
The literature linking childhood obesity with either attention deficit hyperactivity disorder or autism spectrum disorder is complex and the relationship is uncertain. The association seems to be clearer in adults but the mechanisms and their causal directions remain unclear 109 , 197 . Young children with obesity, especially boys, are more likely to be parent-rated as having behavioural problems 198 . This may be a response to the behaviour of others rather than reflect clinical diagnoses such as attention deficit hyperactivity disorder or autism spectrum disorder. Conduct and peer relationship problems co-occur in children, regardless of their weight.
Children with obesity experience more social rejection. They receive fewer friendship nominations and more peer rejections, most pronounced in those with severe obesity 199 . This continues through adolescence and beyond. Children with obesity are more likely to report being victimized 200 . Younger children may respond by being perpetrators themselves. While it is assumed that children are victimized because of their weight, very few studies have looked at the nature or reason behind victimization. A substantial proportion of children with obesity fail to identify themselves as being fat-teased 187 . Although the stigma associated with obesity should be anticipated in children, especially in those most overweight, it would be inappropriate to see all as victims. A better understanding of children’s resilience is needed.
Many gaps remain in basic, translational and clinical research in child and adolescent obesity. The mechanisms (genetic, epigenetic, environmental and social) behind the overwhelming association between parental obesity and child and adolescent obesity are still unclear given the paradoxically weak association in BMI between adopted children and their parents in combination with the modest effect size of known genetic loci associated with obesity 201 .
Early manifestation of extreme obesity in childhood suggests a strong biological basis for disturbances of homeostatic weight regulation. Deep genotyping (including next-generation sequencing) and epigenetic analyses in these patients will reveal new genetic causes and causal pathways as a basis for the development of mechanism-based treatments. Future work aiming to understand the mechanisms underlying the development of childhood obesity should consider the complex biopsychosocial interactions and take a systems approach to understanding causal pathways leading to childhood obesity to contribute to evidence-based prevention and treatment strategies.
Long-term outcome data to better determine the risks of eating disorders are required. Although symptoms improve during obesity treatment in most adolescents, screening and monitoring for disordered eating is recommended in those presenting for treatment 202 and effective tools for use in clinical practice are required. A limited number of tools are validated to identify binge eating disorder in youth with obesity 203 but further research is needed to screen appropriately for the full spectrum of eating disorder diagnoses in obesity treatment seeking youth 203 . Recent reviews provide additional detail regarding eating disorder risk in child and adolescent obesity 117 , 202 , 204 .
Most studies of paediatric obesity treatment have been undertaken in HICs and predominantly middle-class populations. However, research is needed to determine which strategies are best suited for those in LMICs and low-resource settings, for priority population groups including indigenous peoples, migrant populations and those living with social disadvantage, and for children with neurobehavioural and psychiatric disorders. We currently have a limited understanding of how best to target treatment pathways for different levels of genetic risk, age, developmental level, obesity severity, and cardiometabolic and psychological risk. Current outcomes for behavioural interventions are relatively modest and improved treatment outcomes are needed to address the potentially severe long-term health outcomes of paediatric obesity. Studies also need to include longer follow-up periods after an intervention, record all adverse events, incorporate cost-effectiveness analyses and have improved process evaluation.
Other areas in need of research include the role of new anti-obesity medications especially in adolescents, long-term outcomes following bariatric surgery and implementation of digital support systems to optimize outcomes and reduce costs of behavioural change interventions 205 . We must also better understand and tackle the barriers to implementation of treatment in real-life clinical settings, including the role of training of health professionals. Importantly, treatment studies of all kinds must engage people with lived experience — adolescents, parents and families — to understand what outcomes and elements of treatment are most valued.
Obesity prevention is challenging because it requires a multilevel, multisectoral approach that addresses inequity, involves many stakeholders and addresses both the upstream and the downstream factors influencing obesity risk. Some evidence exists of effectiveness of prevention interventions operating at the level of the child, family and school, but the very poor progress overall in modifying obesity prevalence globally highlights many areas in need of research and evidence implementation. Studies are needed especially in LMICs, particularly in the context of the nutrition transition and the double burden of malnutrition. A focus on intergenerational research, rather than the age-based focus of current work, is also needed. Systems research approaches should be used, addressing the broader food and physical activity environments, and links to climate change 206 . In all studies, strategies are needed that enable co-production with relevant communities, long-term follow-up, process evaluation and cost-effectiveness analyses. In the next few years, research and practice priorities must include a focus on intervention strategies in the earliest phases of life, including during pregnancy. The effects of COVID-19 and cost of living crises in many countries are leading to widening health inequalities 207 and this will further challenge obesity prevention interventions. Available resourcing for prevention interventions may become further constrained, requiring innovative solutions across agendas, with clear identification of co-benefits. For example, public health interventions for other diseases, such as dental caries or depression, or other societal concerns, such as urban congestion or climate change, may also act as obesity prevention strategies. Ultimately, to implement obesity prevention, societal changes are needed in terms of urban planning, social structures and health-care access.
Future high-quality paediatric obesity research can be enabled through strategies that support data sharing, which avoids research waste and bias, and enables new research questions to be addressed. Such approaches require leadership, careful engagement of multiple research teams, and resourcing. Four national or regional level paediatric weight registries exist 208 , 209 , 210 , 211 , which are all based in North America or Europe. Such registries should be established in other countries, especially in low-resource settings, even if challenging 208 . Another data-sharing approach is through individual participant data meta-analyses of intervention trials, which can include prospectively collected data 212 and are quite distinct from systematic reviews of aggregate data. Two recent examples are the Transforming Obesity Prevention in Childhood (TOPCHILD) Collaboration, which includes early interventions to prevent obesity in the first 2 years of life 213 , and the Eating Disorders in Weight-Related Therapy (EDIT) Collaboration, which aims to identify characteristics of individuals or trials that increase or protect against eating disorder risk following obesity treatment 214 . Formal data linkage studies, especially those joining up routine administrative datasets, enable longer-term and broader outcome measures to be assessed than is possible with standard clinical or public health intervention studies.
Collaborative research will also be enhanced through the use of agreed core outcome sets, supporting data harmonization. The Edmonton Obesity Staging System – Paediatric 215 is one option for paediatric obesity treatment. A core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH) has been recently established 216 . These efforts incorporate a balance between wanting and needing to share data and adhering to privacy protection regulations. Objective end points are ideal, including directly measured physical activity and body composition.
Collaborative efforts and a systems approach are paramount to understand, prevent and manage child and adolescent obesity. Research funding and health policies should focus on feasible, effective and equitable interventions.
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet https://doi.org/10.1016/S0140-6736(17)32129-3 (2017).
Article Google Scholar
Popkin, B. M., Corvalan, C. & Grummer-Strawn, L. M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395 , 65–74 (2020).
Article PubMed Google Scholar
Kompaniyets, L. et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw. Open 4 , e2111182 (2021).
Article PubMed PubMed Central Google Scholar
Griffiths, L. J., Parsons, T. J. & Hill, A. J. Self‐esteem and quality of life in obese children and adolescents: a systematic review. Int. J. Pediatr. Obes. 5 , 282–304 (2010).
Buttitta, M., Iliescu, C., Rousseau, A. & Guerrien, A. Quality of life in overweight and obese children and adolescents: a literature review. Qual. Life Res. 23 , 1117–1139 (2014).
Hayes, A. et al. Early childhood obesity: association with healthcare expenditure in Australia. Obesity 24 , 1752–1758 (2016).
Marcus, C., Danielsson, P. & Hagman, E. Pediatric obesity – long-term consequences and effect of weight loss. J. Intern. Med. 292 , 870–891 (2022).
Berthoud, H. R., Morrison, C. D. & Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: what went wrong and how can it be fixed? Physiol. Behav. 222 , 112959 (2020).
Article CAS PubMed PubMed Central Google Scholar
World Health Organization. Report of the commission on ending childhood obesity. WHO https://www.who.int/publications/i/item/9789241510066 (2016). This report from the WHO on approaches to childhood and adolescent obesity has six main recommendations for governments, covering food and physical activity, age-based settings and provision of weight management for those with obesity.
O’Connor, E. A. et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 317 , 2427–2444 (2017).
Inge, T. H. et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 380 , 2136–2145 (2019).
Weghuber, D. et al. Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2208601 (2022). To our knowledge, the first RCT of semaglutide 2.4 mg, administered weekly by subcutaneous injection, in adolescents with obesity.
World Health Organization. Report of the Commission on Ending Childhood Obesity: Implementation Plan: Executive Summary (WHO, 2017).
Hillier-Brown, F. C. et al. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health 14 , 834 (2014).
World Health Organization. Obesity. WHO https://www.who.int/health-topics/obesity#tab=tab_1 (2023).
Mei, Z. et al. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 75 , 978–985 (2002).
Article CAS PubMed Google Scholar
World Health Organization. Child growth standards. WHO https://www.who.int/tools/child-growth-standards/standards (2006).
World Health Organization. Growth reference data for 5–19 years. WHO https://www.who.int/tools/growth-reference-data-for-5to19-years (2007).
National Center for Health Statistics. CDC growth charts. Centers for Disease Control and Prevention http://www.cdc.gov/growthcharts/ (2022).
Cole, T. J., Bellizzi, M. C., Flegal, K. M. & Dietz, W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320 , 1240 (2000).
Di Cesare, M. et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 17 , 212 (2019).
Swinburn, B., Egger, G. & Raza, F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev. Med. 29 , 563–570 (1999).
Ford, N. D., Patel, S. A. & Narayan, K. V. Obesity in low-and middle-income countries: burden, drivers, and emerging challenges. Annu. Rev. Public Health 38 , 145–164 (2017).
Shah, B., Tombeau Cost, K., Fuller, A., Birken, C. S. & Anderson, L. N. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr. Prev. Health 3 , 387–390 (2020).
Public Health England. Research and analysis: differences in child obesity by ethnic group. GOV.UK https://www.gov.uk/government/publications/differences-in-child-obesity-by-ethnic-group/differences-in-child-obesity-by-ethnic-group#data (2019).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr. Obes. 7 , 284–294 (2012).
Kelly, A. S. et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 128 , 1689–1712 (2013).
Garnett, S. P., Baur, L. A., Jones, A. M. & Hardy, L. L. Trends in the prevalence of morbid and severe obesity in Australian children aged 7–15 years, 1985-2012. PLoS ONE 11 , e0154879 (2016).
Spinelli, A. et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes. Facts 12 , 244–258 (2019).
Twig, G. et al. Body-Mass Index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 374 , 2430–2440 (2016).
González-Muniesa, P. et al. Obesity. Nat. Rev. Dis. Primers 3 , 17034 (2017).
Geserick, M. et al. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 379 , 1303–1312 (2018).
Larqué, E. et al. From conception to infancy – early risk factors for childhood obesity. Nat. Rev. Endocrinol. 15 , 456–478 (2019).
Barker, D. J. Fetal origins of coronary heart disease. Br. Med. J. 311 , 171–174 (1995).
Article CAS Google Scholar
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359 , 61–73 (2008).
Philips, E. M. et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: an individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 17 , e1003182 (2020).
Voerman, E. et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 16 , e1002744 (2019). Individual participant data meta-analysis of >160,000 mothers and their children on the associations of maternal BMI and gestational weight gain and childhood overweight or obesity.
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5 , 47 (2019).
Oken, E. & Gillman, M. W. Fetal origins of obesity. Obes. Res. 11 , 496–506 (2003).
Hughes, A. R., Sherriff, A., Ness, A. R. & Reilly, J. J. Timing of adiposity rebound and adiposity in adolescence. Pediatrics 134 , e1354–e1361 (2014).
Rolland-Cachera, M. F. et al. Tracking the development of adiposity from one month of age to adulthood. Ann. Hum. Biol. 14 , 219–229 (1987).
Koletzko, B. et al. Prevention of childhood obesity: a position paper of the Global Federation of International Societies of Paediatric Gastroenterology, Hepatology and Nutrition (FISPGHAN). J. Pediatr. Gastroenterol. Nutr. 70 , 702–710 (2020).
Weber, M. et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am. J. Clin. Nutr. 99 , 1041–1051 (2014).
Cope, M. B. & Allison, D. B. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long‐term effects of breastfeeding: systematic reviews and meta‐analysis’ with respect to obesity. Obes. Rev. 9 , 594–605 (2008).
Totzauer, M. et al. Different protein intake in the first year and its effects on adiposity rebound and obesity throughout childhood: 11 years follow‐up of a randomized controlled trial. Pediatr. Obes. 17 , e12961 (2022).
Deren, K. et al. Consumption of sugar-sweetened beverages in paediatric age: a position paper of the European academy of paediatrics and the European Childhood Obesity Group. Ann. Nutr. Metab. 74 , 296–302 (2019).
Felső, R., Lohner, S., Hollódy, K., Erhardt, É. & Molnár, D. Relationship between sleep duration and childhood obesity: systematic review including the potential underlying mechanisms. Nutr. Metab. Cardiovasc. Dis. 27 , 751–761 (2017).
Farooq, A. et al. Longitudinal changes in moderate‐to‐vigorous‐intensity physical activity in children and adolescents: a systematic review and meta‐analysis. Obes. Rev. 21 , e12953 (2020).
Mahumud, R. A. et al. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int. J. Obes. 45 , 2404–2418 (2021).
Ballon, M. et al. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int. J. Obes. 42 , 1671–1679 (2018).
Buoncristiano, M. et al. Socioeconomic inequalities in overweight and obesity among 6- to 9-year-old children in 24 countries from the World Health Organization European region. Obes. Rev. 22 , e13213 (2021).
Jiwani, S. S. et al. The shift of obesity burden by socioeconomic status between 1998 and 2017 in Latin America and the Caribbean: a cross-sectional series study. Lancet Glob. Health 7 , e1644–e1654 (2019).
Monteiro, C. A., Conde, W. L., Lu, B. & Popkin, B. M. Obesity and inequities in health in the developing world. Int. J. Obes. 28 , 1181–1186 (2004).
Guo, S. S. & Chumlea, W. C. Tracking of body mass index in children in relation to overweight in adulthood. Am. J. Clin. Nutr. 70 , 145S–148S (1999).
Aarestrup, J. et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int. J. Obes. 44 , 1546–1560 (2020).
Eslam, M. et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol. Hepatol. 6 , 864–873 (2021).
Daniels, S. R. et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 111 , 1999–2012 (2005).
Cioana, M. et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw. Open 5 , e2247186 (2022).
Gepstein, V. & Weiss, R. Obesity as the main risk factor for metabolic syndrome in children. Front. Endocrinol. 10 , 568 (2019).
Kuvat, N., Tanriverdi, H. & Armutcu, F. The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin. Respir. J. 14 , 595–604 (2020).
Baker, J. L., Olsen, L. W. & Sorensen, T. I. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 357 , 2329–2337 (2007).
Bjerregaard, L. G. et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 378 , 1302–1312 (2018).
Kelsey, M. M., Zaepfel, A., Bjornstad, P. & Nadeau, K. J. Age-related consequences of childhood obesity. Gerontology 60 , 222–228 (2014).
Sharma, V. et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes. Rev. 20 , 1341–1349 (2019).
Lobstein, T. & Jackson-Leach, R. Planning for the worst: estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 11 , 321–325 (2016).
Berthoud, H. R., Münzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152 , 1728–1738 (2017).
Devoto, F. et al. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 94 , 271–285 (2018).
Blum, W. F., Englaro, P., Attanasio, A. M., Kiess, W. & Rascher, W. Human and clinical perspectives on leptin. Proc. Nutr. Soc. 57 , 477–485 (1998).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1 , 754–764 (2019).
Kühnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016).
Clément, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156 , 20–44 (2014).
Fischer-Posovszky, P., Roos, J., Zoller, V. & Wabitsch, M. in Pediatric Obesity: Etiology, Pathogenesis and Treatment (ed. Freemark, M. S.) 81–93 (Springer, 2018).
Hammarstedt, A., Gogg, S., Hedjazifar, S., Nerstedt, A. & Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98 , 1911–1941 (2018).
Silventoinen, K. et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the collaborative project of development of anthropometrical measures in twins (CODATwins) study. Am. J. Clin. Nutr. 104 , 371–379 (2016).
Silventoinen, K. et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. Am. J. Clin. Nutr. 106 , 457–466 (2017).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019). To our knowledge, currently the largest genome-wide association study meta-analysis on childhood obesity in >13,000 individuals with obesity and >15,500 controls.
Couto Alves, A. et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 5 , eaaw3095 (2019).
Ding, X. et al. Genome-wide screen of DNA methylation identifies novel markers in childhood obesity. Gene 566 , 74–83 (2015).
Huang, R. C. et al. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics 10 , 995–1005 (2015).
Rzehak, P. et al. DNA-methylation and body composition in preschool children: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP)-Study. Sci. Rep. 7 , 14349 (2017).
Alfano, R. et al. Perspectives and challenges of epigenetic determinants of childhood obesity: a systematic review. Obes. Rev. 23 , e13389 (2022).
Vehmeijer, F. O. L. et al. DNA methylation and body mass index from birth to adolescence: meta-analyses of epigenome-wide association studies. Genome Med. 12 , 105 (2020). Meta-analysis of epigenome-wide association studies of childhood BMI in >4,000 children.
Richmond, R. C. et al. DNA methylation and BMI: investigating identified methylation sites at HIF3A in a causal framework. Diabetes 65 , 1231–1244 (2016).
Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541 , 81–86 (2017).
Kivimäki, M. et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am. J. Clin. Nutr. 86 , 1509–1514 (2007).
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337 , 869–873 (1997).
Davey Smith, G., Steer, C., Leary, S. & Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch. Dis. Child. 92 , 876–880 (2007).
Fleten, C. et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am. J. Epidemiol. 176 , 83–92 (2012).
Gaillard, R. et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63 , 683–691 (2014).
Lawlor, D. A. et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 5 , e33 (2008).
Patro, B. et al. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann. Nutr. Metab. 63 , 32–41 (2013).
Sorensen, T. et al. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7 y assessed in the Danish National Birth Cohort. Am. J. Clin. Nutr. 104 , 389–396 (2016).
Styne, D. M. et al. Pediatric obesity – assessment, treatment, and prevention: an Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 102 , 709–757 (2017).
National Institute for Health and Care Excellence. Obesity: identification, assessment and managenent: clinical guideline [CG189]. NICE https://www.nice.org.uk/guidance/cg189 (2022). A high quality clinical practice guideline for obesity management.
Barlow, S. E. Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 (Suppl. 4), S164–S192 (2007).
Canadian Task Force on Preventive Health Care. Recommendations for growth monitoring, and prevention and management of overweight and obesity in children and youth in primary care. Can. Med. Assoc. J. 187 , 411–421 (2015).
US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 317 , 2417–2426 (2017).
McConnell-Nzunga, J. et al. Classification of obesity varies between body mass index and direct measures of body fat in boys and girls of Asian and European ancestry. Meas. Phys. Educ. Exerc. Sci. 22 , 154–166 (2018).
Reinehr, T. et al. Definable somatic disorders in overweight children and adolescents. J. Pediatr. 150 , 618–622.e5 (2007).
Reinehr, T. Thyroid function in the nutritionally obese child and adolescent. Curr. Opin. Pediatr. 23 , 415–420 (2011).
Kohlsdorf, K. et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int. J. Obes. 42 , 1602–1609 (2018).
Armstrong, S. et al. Physical examination findings among children and adolescents with obesity: an evidence-based review. Pediatrics 137 , e20151766 (2016).
Reinehr, T. & Roth, C. L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc. Health 3 , 44–54 (2019).
Garnett, S. P., Baur, L. A. & Cowell, C. T. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int. J. Obes. 32 , 1028–1030 (2008).
Maffeis, C., Banzato, C., Talamini, G. & Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 152 , 207–213.e2 (2008).
Hampl, S. E. et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 151 , e2022060640 (2023). A new, comprehensive clinical practice guideline outlining current recommendations on assessment and treatment of children and adolescents with obesity.
Reinehr, T. et al. Comparison of cardiovascular risk factors between children and adolescents with classes III and IV obesity: findings from the APV cohort. Int. J. Obes. 45 , 1061–1073 (2021).
Reinehr, T. Metabolic syndrome in children and adolescents: a critical approach considering the interaction between pubertal stage and insulin resistance. Curr. Diabetes Rep. 16 , 8 (2016).
Zeitler, P. et al. ISPAD Clinical Practice Consensus Guidelines 2018: type 2 diabetes mellitus in youth. Pediatr. Diabetes 19 , 28–46 (2018).
Ibáñez, L. et al. An International Consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 88 , 371–395 (2017).
Brockmann, P. E., Schaefer, C., Poets, A., Poets, C. F. & Urschitz, M. S. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med. Rev. 17 , 331–340 (2013).
Taylor, E. D. et al. Orthopedic complications of overweight in children and adolescents. Pediatrics 117 , 2167–2174 (2006).
Winck, A. D. et al. Effects of obesity on lung volume and capacity in children and adolescents: a systematic review. Rev. Paul. Pediatr. 34 , 510–517 (2016).
PubMed PubMed Central Google Scholar
Jebeile, H., Lister, N., Baur, L., Garnett, S. & Paxton, S. J. Eating disorder risk in adolescents with obesity. Obes. Rev. 22 , e13173 (2021).
Quek, Y. H., Tam, W. W. S., Zhang, M. W. B. & Ho, R. C. M. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes. Rev. 18 , 742–754 (2017).
World Health Organization. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity . Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity (WHO, 2016).
Pickett, K. et al. The Child of the North: building a fairer future after COVID-19. The Northern Health Science Alliance and N8 Research Partnership https://www.thenhsa.co.uk/app/uploads/2022/01/Child-of-the-North-Report-FINAL-1.pdf (2021).
Bronfenbrenner, U. Toward an experimental ecology of human development. Am. Psychol. 32 , 513–531 (1977).
Nuffield Council on Bioethics. Public Health: Ethical Issues (Nuffield Council on Bioethics, 2007).
Lorenc, T., Petticrew, M., Welch, V. & Tugwell, P. What types of interventions generate inequalities? Evidence from systematic reviews. J. Epidemiol. Community Health 67 , 190–193 (2013).
Adams, J., Mytton, O., White, M. & Monsivais, P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 13 , e1001990 (2016).
Backholer, K. et al. A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am. J. Public Health 104 , e43–e50 (2014).
McGill, R. et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health 15 , 457 (2015).
Brown, T. et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 7 , Cd001871 (2019). A Cochrane review involving 153 RCTs of diet and/or physical activity interventions to prevent obesity in children and adolescents, highlighting varying effectiveness of interventions in different age groups.
PubMed Google Scholar
Le, L. K.-D. et al. Prevention of high body mass index and eating disorders: a systematic review and meta-analysis. Eat. Weight Disord. 27 , 2989–3003 (2022).
Nobles, J., Summerbell, C., Brown, T., Jago, R. & Moore, T. A secondary analysis of the childhood obesity prevention Cochrane Review through a wider determinants of health lens: implications for research funders, researchers, policymakers and practitioners. Int. J. Behav. Nutr. Phys. Act. 18 , 22 (2021).
Rai, K. K., Dogra, S. A., Barber, S., Adab, P. & Summerbell, C. A scoping review and systematic mapping of health promotion interventions associated with obesity in Islamic religious settings in the UK. Obes. Rev. 20 , 1231–1261 (2019).
World Health Organization. Nutrition Action in Schools: A Review of Evidence Related to the Nutrition-Friendly Schools Initiative (WHO, 2021).
Daly-Smith, A. et al. Using a multi-stakeholder experience-based design process to co-develop the Creating Active Schools Framework. Int. J. Behav. Nutr. Phys. Act. 17 , 13 (2020).
Tibbitts, B. et al. Considerations for individual-level versus whole-school physical activity interventions: stakeholder perspectives. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph18147628 (2021).
Askie, L. M. et al. Interventions commenced by early infancy to prevent childhood obesity-The EPOCH Collaboration: an individual participant data prospective meta-analysis of four randomized controlled trials. Pediatr. Obes. 15 , e12618 (2020). To our knowledge, the first prospective individual participant data meta-analysis showing that interventions commencing in late pregnancy or very early childhood are associated with healthier BMI z -score at age 18–24 months.
Seidler, A. L. et al. Examining the sustainability of effects of early childhood obesity prevention interventions: follow-up of the EPOCH individual participant data prospective meta-analysis. Pediatr. Obes. 17 , e12919 (2022).
Taylor, R. W. et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the prevention of overweight in infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am. J. Clin. Nutr. 108 , 228–236 (2018).
Mihrshahi, S. et al. A review of registered randomized controlled trials for the prevention of obesity in infancy. Int. J. Environ. Res. Public Health 18 , 2444 (2021).
Farooq, M. A. et al. Timing of the decline in physical activity in childhood and adolescence: Gateshead Millennium Cohort Study. Br. J. Sports Med. 52 , 1002–1006 (2018).
van Sluijs, E. M. F. et al. Physical activity behaviours in adolescence: current evidence and opportunities for intervention. Lancet 398 , 429–442 (2021).
Griffin, N. et al. A critique of the English national policy from a social determinants of health perspective using a realist and problem representation approach: the ‘Childhood Obesity: a plan for action’ (2016, 2018, 2019). BMC Public Health 21 , 2284 (2021).
Knai, C., Lobstein, T., Petticrew, M., Rutter, H. & Savona, N. England’s childhood obesity action plan II. Br. Med. J. 362 , k3098 (2018).
World Health Organization. WHO European Regional Obesity Report 2022 (WHO, 2022).
Zemrani, B., Gehri, M., Masserey, E., Knob, C. & Pellaton, R. A hidden side of the COVID-19 pandemic in children: the double burden of undernutrition and overnutrition. Int. J. Equity Health 20 , 44 (2021).
Alman, K. L. et al. Dietetic management of obesity and severe obesity in children and adolescents: a scoping review of guidelines. Obes. Rev. https://doi.org/10.1111/obr.13132 (2020).
Pfeiffle, S. et al. Current recommendations for nutritional management of overweight and obesity in children and adolescents: a structured framework. Nutrients https://doi.org/10.3390/nu11020362 (2019).
Scottish Intercollegiate Guidelines Network. Management of obesity. A National Clinical Guideline . SIGN 115 (SIGN, 2010).
Reinehr, T. et al. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity 17 , 1196–1199 (2009).
Ells, L. J. et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int. J. Obes. 42 , 1823–1833 (2018).
Al‐Khudairy, L. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 6 , CD012691 (2017). One of three Cochrane reviews looking at lifestyle treatment of paediatric obesity, in this case in adolescents, which identified 44 completed trials, finding low quality evidence of improvements in BMI and moderate quality evidence of improvements in weight.
Mead, E. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 6 , CD012651 (2017). A Cochrane Review, involving 70 RCTs, showing that multicomponent behavioural interventions can lead to small, short-term reductions in BMI and related measures in children aged 6–11 years with obesity.
Ho, M. et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics 130 , e1647–e1671 (2012). To our knowledge, the first systematic review of lifestyle interventions in children and adolescents with obesity to show improvements in cardiometabolic outcomes (LDL cholesterol, triglycerides, fasting insulin and blood pressure), as well as weight.
Clinical Practice Guideline Panel. Clinical practice guideline for multicomponent behavioral treatment of obesity and overweight in children and adolescents: current state of the evidence and research needs. American Psychological Association https://www.apa.org/obesity-guideline/clinical-practice-guideline.pdf (2018).
Nowicka, P. & Flodmark, C. E. Family therapy as a model for treating childhood obesity: useful tools for clinicians. Clin. Child. Psychol. Psychiatry 16 , 129–145 (2011).
Wilfley, D. E. et al. Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity 25 , 16–29 (2017).
Amiri, P. et al. Does motivational interviewing improve the weight management process in adolescents? A systematic review and meta-analysis. Int. J. Behav. Med. 29 , 78–103 (2022).
Kao, T. A., Ling, J., Hawn, R. & Vu, C. The effects of motivational interviewing on children’s body mass index and fat distributions: a systematic review and meta-analysis. Obes. Rev. 22 , e13308 (2021).
Hassapidou, M. et al. European Association for the Study of Obesity (EASO) position statement on medical nutrition therapy for the management of overweight and obesity in children and adolescents developed in collaboration with the European Federation of the Associations of Dietitians (EFAD). Obes. Facts https://doi.org/10.1159/000527540 (2022).
Hoelscher, D. M., Kirk, S., Ritchie, L. & Cunningham-Sabo, L. Position of the Academy of Nutrition and Dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. J. Acad. Nutr. Diet. 113 , 1375–1394 (2013).
Hoare, J. K., Jebeile, H., Garnett, S. P. & Lister, N. B. Novel dietary interventions for adolescents with obesity: a narrative review. Pediatr. Obes. 16 , e12798 (2021).
Lister, N. et al. Nutritional adequacy of diets for adolescents with overweight and obesity: considerations for dietetic practice. Eur. J. Clin. Nutr. 71 , 646–651 (2017).
Andela, S. et al. Efficacy of very low-energy diet programs for weight loss: a systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes. Rev. 20 , 871–882 (2019).
Srivastava, G. & Apovian, C. M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 14 , 12–24 (2018).
Apperley, L. J. et al. Childhood obesity: a review of current and future management options. Clin. Endocrinol. 96 , 288–301 (2022).
European Medicines Agency. Saxenda. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (2022).
US Food and Drug Administration. FDA approves weight management drug for patients aged 12 and older. FDA https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older (2021).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382 , 2117–2128 (2020). To our knowledge, the first RCT of liraglutide, administered daily via subcutaneous injection, in adolescents with obesity.
US Food and Drug Administration. FDA approves novel, dual-targeted treatment for type 2 iabetes. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes (2022).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387 , 205–216 (2022).
Holst, J. J. & Rosenkilde, M. M. GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaa327 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT05260021 (2023).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21 , 201–223 (2022).
Chalklin, C. G., Ryan Harper, E. G. & Beamish, A. J. Metabolic and bariatric surgery in adolescents. Curr. Obes. Rep. 10 , 61–69 (2021).
Albaugh, V. L. et al. Regulation of body weight: lessons learned from bariatric surgery. Mol. Metab. https://doi.org/10.1016/j.molmet.2022.101517 (2022).
Uhe, I. et al. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity 30 , 614–627 (2022).
Inge, T. H. et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 5 , 165–173 (2017).
Olbers, T. et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(16)30424-7 (2017).
Pratt, J. S. et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 14 , 882–901 (2018).
Jarvholm, K. et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc. Health 4 , 210–219 (2020).
Van Der Heijden, L., Feskens, E. & Janse, A. Maintenance interventions for overweight or obesity in children: a systematic review and meta‐analysis. Obes. Rev. 19 , 798–809 (2018).
Park, J., Park, M.-J. & Seo, Y.-G. Effectiveness of information and communication technology on obesity in childhood and adolescence: systematic review and meta-analysis. J. Med. Internet Res. 23 , e29003 (2021).
Brissman, M., Beamish, A. J., Olbers, T. & Marcus, C. Prevalence of insufficient weight loss 5 years after Roux-en-Y gastric bypass: metabolic consequences and prediction estimates: a prospective registry study. BMJ Open 11 , e046407 (2021).
El Ansari, W. & Elhag, W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps – a scoping review. Obes. Surg. 31 , 1755–1766 (2021).
World Health Organization Regional Office for Europe. Weight Bias and Obesity Stigma: Considerations for the WHO European Region (WHO, 2017).
Puhl, R. M. & Latner, J. D. Stigma, obesity, and the health of the nation’s children. Psychol. Bull. 133 , 557–580 (2007).
Black, W. R. et al. Health-related quality of life across recent pediatric obesity classification recommendations. Children 8 , 303 (2021).
Finne, E., Reinehr, T., Schaefer, A., Winkel, K. & Kolip, P. Changes in self-reported and parent-reported health-related quality of life in overweight children and adolescents participating in an outpatient training: findings from a 12-month follow-up study. Health Qual. Life Outcomes 11 , 1 (2013).
Hill, A. J. Obesity in children and the ‘myth of psychological maladjustment’: self-esteem in the spotlight. Curr. Obes. Rep. 6 , 63–70 (2017).
McGregor, S., McKenna, J., Gately, P. & Hill, A. J. Self‐esteem outcomes over a summer camp for obese youth. Pediatr. Obes. 11 , 500–505 (2016).
Jansen, P., Mensah, F., Clifford, S., Nicholson, J. & Wake, M. Bidirectional associations between overweight and health-related quality of life from 4–11 years: longitudinal study of Australian children. Int. J. Obes. 37 , 1307–1313 (2013).
Mannan, M., Mamun, A., Doi, S. & Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatr. 21 , 51–66 (2016).
Lindberg, L., Hagman, E., Danielsson, P., Marcus, C. & Persson, M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 18 , 30 (2020).
Jebeile, H. et al. Association of pediatric obesity treatment, including a dietary component, with change in depression and anxiety: a systematic review and meta-analysis. JAMA Pediatr. 173 , e192841 (2019).
Gow, M. L. et al. Pediatric obesity treatment, self‐esteem, and body image: a systematic review with meta‐analysis. Pediatr. Obes. 15 , e12600 (2020).
Jebeile, H. et al. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: a systematic review with meta-analysis. Obes. Rev. 20 , 1287–1298 (2019). To our knowledge, the first systematic review to show that structured and professionally led weight management interventions in children and adolescents with obesity are associated with reductions in eating disorder risk and symptoms.
Kjeldbjerg, M. L. & Clausen, L. Prevalence of binge-eating disorder among children and adolescents: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01850-2 (2021).
Patton, G. C., Selzer, R., Coffey, C., Carlin, J. B. & Wolfe, R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ 318 , 765–768 (1999).
Cortese, S. The association between ADHD and obesity: intriguing, progressively more investigated, but still puzzling. Brain Sci. 9 , 256 (2019).
Griffiths, L. J., Dezateux, C. & Hill, A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. Int. J. Pediatr. Obes. 6 , e423–e432 (2011).
Harrist, A. W. et al. The social and emotional lives of overweight, obese, and severely obese children. Child. Dev. 87 , 1564–1580 (2016).
Van Geel, M., Vedder, P. & Tanilon, J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the relation between weight status and bullying. Int. J. Obes. 38 , 1263–1267 (2014).
Albuquerque, D., Nóbrega, C., Manco, L. & Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 123 , 159–173 (2017).
Rancourt, D. & McCullough, M. B. Overlap in eating disorders and obesity in adolescence. Curr. Diabetes Rep. 15 , 78 (2015).
House, E. T. et al. Identifying eating disorders in adolescents and adults with overweight or obesity: a systematic review of screening questionnaires. Int. J. Eat. Disord. 55 , 1171–1193 (2022).
Lister, N. B., Baur, L. A., Paxton, S. J. & Jebeile, H. Contextualising eating disorder concerns for paediatric obesity treatment. Curr. Obes. Rep. 10 , 322–331 (2021).
Hagman, E. et al. Effect of an interactive mobile health support system and daily weight measurements for pediatric obesity treatment, a 1-year pragmatical clinical trial. Int. J. Obes. 46 , 1527–1533 (2022).
Swinburn, B. A. et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet commission report. Lancet 393 , 791–846 (2019).
Whitehead, M., Taylor-Robinson, D. & Barr, B. Poverty, health, and covid-19. Br. Med. J. 372 , n376 (2021).
Morrison, K. M. et al. The CANadian Pediatric Weight Management Registry (CANPWR): lessons learned from developing and initiating a national, multi-centre study embedded in pediatric clinical practice. BMC Pediatr. 18 , 237 (2018).
Kirk, S. et al. Establishment of the pediatric obesity weight evaluation registry: a national research collaborative for identifying the optimal assessment and treatment of pediatric obesity. Child. Obes. 13 , 9–17 (2017).
Hagman, E., Danielsson, P., Lindberg, L. & Marcus, C., BORIS Steering Committee. Paediatric obesity treatment during 14 years in Sweden: lessons from the Swedish Childhood Obesity Treatment Register – BORIS. Pediatr. Obes. 15 , e12626 (2020).
Bohn, B. et al. Changing characteristics of obese children and adolescents entering pediatric lifestyle intervention programs in Germany over the last 11 years: an adiposity patients registry multicenter analysis of 65,453 children and adolescents. Obes. Facts 10 , 517–530 (2017).
Seidler, A. L. et al. A guide to prospective meta-analysis. BMJ 367 , l5342 (2019).
Hunter, K. E. et al. Transforming obesity prevention for children (TOPCHILD) collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioural interventions for the prevention of early childhood obesity. BMJ Open 12 , e048166 (2022).
Lister, N. B. et al. Eating disorders in weight-related therapy (EDIT) collaboration: rationale and study design. Nutr. Res. Rev. https://doi.org/10.1017/S0954422423000045 (2023).
Hadjiyannakis, S. et al. Obesity class versus the Edmonton Obesity Staging System for Pediatrics to define health risk in childhood obesity: results from the CANPWR cross-sectional study. Lancet Child Adolesc. Health 3 , 398–407 (2019).
Brown, V. et al. Core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH): agreement on “what” to measure. Int. J. Obes. 46 , 1867–1874 (2022). A stakeholder-informed study that identified the minimum outcomes recommended for collecting and reporting in obesity prevention trials in early childhood.
Han, J. C., Lawlor, D. A. & Kimm, S. Y. Childhood obesity. Lancet 375 , 1737–1748 (2010).
Shin, A. C., Zheng, H. & Berthoud, H. R. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol. Behav. 97 , 572–580 (2009).
Lennerz, B., Wabitsch, M. & Eser, K. Ätiologie und genese [German]. Berufl. Rehabil. 1 , 14 (2014).
Google Scholar
Pont, S. J., Puhl, R., Cook, S. R. & Slusser, W. Stigma experienced by children and adolescents with obesity. Pediatrics 140 , e20173034 (2017).
Talumaa, B., Brown, A., Batterham, R. L. & Kalea, A. Z. Effective strategies in ending weight stigma in healthcare. Obes. Rev. 23 , e13494 (2022).
Rubino, F. et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 26 , 485–497 (2020).
Download references
Author information
Authors and affiliations.
Children’s Hospital Westmead Clinical School, Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia
Natalie B. Lister & Louise A. Baur
Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
Natalie B. Lister
Sydney School of Public Health, The University of Sydney, Sydney, New South Wales, Australia
Louise A. Baur
Weight Management Services, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Janine F. Felix
Department of Paediatrics, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Institute of Health Sciences, School of Medicine, University of Leeds, Leeds, UK
Andrew J. Hill
Division of Paediatrics, Department of Clinical Science Intervention and Technology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden
Claude Marcus
Vestische Hospital for Children and Adolescents Datteln, University of Witten/Herdecke, Datteln, Germany
Thomas Reinehr
Department of Sport and Exercise Sciences, Durham University, Durham, UK
- Carolyn Summerbell
Division of Paediatric Endocrinology and Diabetes, Department of Paediatrics and Adolescent Medicine, Ulm University Medical Centre, Ulm, Germany
Martin Wabitsch
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (L.A.B., J.F.F. and N.B.L.); Epidemiology (L.A.B. and J.F.F.); Mechanisms/pathophysiology (L.A.B., J.F.F., T.R. and M.W.); Diagnosis, screening and prevention (L.A.B., N.B.L., T.R., C.S. and M.W.); Management (L.A.B., N.B.L., A.J.H., C.M. and T.R.); Quality of life (L.A.B., N.B.L. and A.J.H.); Outlook (L.A.B., N.B.L., J.F.F., A.J.H., C.M., T.R., C.S. and M.W.); Overview of the Primer (L.A.B. and N.B.L.).
Corresponding author
Correspondence to Louise A. Baur .
Ethics declarations
Competing interests.
A.J.H. reports receiving payment for consultancy advice for Slimming World (UK). L.A.B. reports receiving honoraria for speaking in forums organized by Novo Nordisk in relation to management of adolescent obesity and the ACTION-Teens study, which is sponsored by Novo Nordisk. L.A.B. is the Australian lead of the study. T.R. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). T.R. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk and Lilly, as well as honoraria for lectures in symposia organized by Novo Nordisk, Novartis and Merck. C.M. receives payments for consultancy advice and advisory board participation from Novo Nordisk, Oriflame Wellness, DeFaire AB and Itrim AB. C.M. also receives honoraria for speaking at meetings organized by Novo Nordisk and Astra Zeneca. C.M. is a shareholder and founder of Evira AB, a company that develops and sells systems for digital support for weight loss, and receives grants from Novo Nordisk for epidemiological studies of the effects of weight loss on future heath. M.W. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). M.W. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk, Regeneron, Boehringer Ingelheim and LG Chem, as well as honoraria for speaking in symposia organized by Novo Nordisk, Rhythm Pharmaceuticals and Infectopharm. M.W. is principal investigator in phase II and phase III studies of setmelanotide sponsored by Rhythm Pharmaceuticals. N.B.L., J.F.F. and C.S. declare no competing interests.
Peer review
Peer review information.
Nature Reviews Disease Primers thanks C. Maffeis, L. Moreno, R, Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lister, N.B., Baur, L.A., Felix, J.F. et al. Child and adolescent obesity. Nat Rev Dis Primers 9 , 24 (2023). https://doi.org/10.1038/s41572-023-00435-4
Download citation
Accepted : 12 April 2023
Published : 18 May 2023
DOI : https://doi.org/10.1038/s41572-023-00435-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Relationship between 24-h activity behavior and body fat percentage in preschool children: based on compositional data and isotemporal substitution analysis.
BMC Public Health (2024)
Young people's experiences of physical activity insecurity: a qualitative study highlighting intersectional disadvantage in the UK
- Caroline Dodd-Reynolds
- Naomi Griffin
Enough with simplifying: “eat less and move more”: at what point are we with the treatment of excess weight in paediatrics?
- Giovanni Corsello
- Sergio Bernasconi
Italian Journal of Pediatrics (2024)
Association between watching eating broadcast “Mukbang and Cookbang” and body mass index status in South Korean adolescents stratified by gender
- Sang-yeon Park
- Jeongha Eom
- Eun-Cheol Park
Nutrition Journal (2024)
The relationship between body mass index and cerebrospinal fluid pressure in children with pseudotumor cerebri
- Safiye Güneş Sağer
- Yasemin Akın
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Childhood obesity: A review of current and future management options
Affiliations.
- 1 Department of Paediatric Endocrinology, Alder Hey Children's Hospital, Liverpool, UK.
- 2 Centre for Endocrinology, William Harvey Research Institute, Barts and the London School of Medicine, Queen Mary University of London, London, UK.
- 3 Department of Paediatric Dietetics, Alder Hey Children's Hospital, Liverpool, UK.
- 4 Department of Paediatric Clinical Psychology, Alder Hey Children's Hospital, Liverpool, UK.
- PMID: 34750858
- DOI: 10.1111/cen.14625
Obesity is becoming increasingly prevalent in paediatric populations worldwide. In addition to increasing prevalence, the severity of obesity is also continuing to rise. Taken together, these findings demonstrate a worrying trend and highlight one of the most significant challenges to public health. Childhood obesity affects multiple organs in the body and is associated with both significant morbidity and ultimately premature mortality. The prevalence of complications associated with obesity, including dyslipidaemia, hypertension, fatty liver disease and psychosocial complications are becoming increasingly prevalent within the paediatric populations. Treatment guidelines currently focus on intervention with lifestyle and behavioural modifications, with pharmacotherapy and surgery reserved for patients who are refractory to such treatment. Research into adult obesity has established pharmacological novel therapies, which have been approved and established in clinical practice; however, the research and implementation of such therapies in paediatric populations have been lagging behind. Despite the relative lack of widespread research in comparison to the adult population, newer therapies are being trialled, which should allow a greater availability of treatment options for childhood obesity in the future. This review summarizes the current evidence for the management of obesity in terms of medical and surgical options. Both future therapeutic agents and those which cause weight loss but have an alternative indication are also included and discussed as part of the review. The review summarizes the most recent research for each intervention and demonstrates the potential efficacy and limitations of each treatment option.
Keywords: BMI; childhood obesity; lifestyle interventions; paediatrics; pharmacotherapy.
© 2021 John Wiley & Sons Ltd.
PubMed Disclaimer
Similar articles
- The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review. Antwi F, Fazylova N, Garcon MC, Lopez L, Rubiano R, Slyer JT. Antwi F, et al. JBI Libr Syst Rev. 2012;10(42 Suppl):1-14. doi: 10.11124/jbisrir-2012-248. JBI Libr Syst Rev. 2012. PMID: 27820152
- Treatment Options for Severe Obesity in the Pediatric Population: Current Limitations and Future Opportunities. Ryder JR, Fox CK, Kelly AS. Ryder JR, et al. Obesity (Silver Spring). 2018 Jun;26(6):951-960. doi: 10.1002/oby.22196. Epub 2018 May 7. Obesity (Silver Spring). 2018. PMID: 29732716 Review.
- The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
- Real-World Strategies to Treat Hypertension Associated with Pediatric Obesity. Binka E, Brady TM. Binka E, et al. Curr Hypertens Rep. 2019 Feb 12;21(2):18. doi: 10.1007/s11906-019-0922-2. Curr Hypertens Rep. 2019. PMID: 30747290 Free PMC article. Review.
- Screening and Interventions for Childhood Overweight [Internet]. Whitlock EP, Williams SB, Gold R, Smith P, Shipman S. Whitlock EP, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Jul. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005 Jul. PMID: 20722132 Free Books & Documents. Review.
- Is oxidative stress - antioxidants imbalance the physiopathogenic core in pediatric obesity? Lupu A, Fotea S, Jechel E, Starcea IM, Ioniuc I, Knieling A, Salaru DL, Sasaran MO, Cirstea O, Revenco N, Mihai CM, Lupu VV, Nedelcu AH. Lupu A, et al. Front Immunol. 2024 Aug 8;15:1394869. doi: 10.3389/fimmu.2024.1394869. eCollection 2024. Front Immunol. 2024. PMID: 39176098 Free PMC article. Review.
- The Prevention of Childhood Obesity Is a Priority: The Preliminary Results of the "EpPOI: Education to Prevent Childhood Obesity" Project. Porri D, Luppino G, Morabito LA, La Rosa E, Pepe G, Corica D, Valenzise M, Messina MF, Zirilli G, Li Pomi A, Alibrandi A, Di Mauro D, Aversa T, Wasniewska MG. Porri D, et al. Nutrients. 2024 Aug 2;16(15):2538. doi: 10.3390/nu16152538. Nutrients. 2024. PMID: 39125417 Free PMC article.
- Comparative Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity or Overweight: A Systematic Review and Network Meta-Analysis. Liu L, Shi H, Shi Y, Wang A, Guo N, Tao H, Nahata MC. Liu L, et al. Pharmaceuticals (Basel). 2024 Jun 24;17(7):828. doi: 10.3390/ph17070828. Pharmaceuticals (Basel). 2024. PMID: 39065679 Free PMC article. Review.
- Analysis of Prescription Trends for Narcotic Appetite Suppressants: Utilizing the Narcotics Information Management System. Oh KS, Han E. Oh KS, et al. Yonsei Med J. 2024 Aug;65(8):480-487. doi: 10.3349/ymj.2023.0335. Yonsei Med J. 2024. PMID: 39048324 Free PMC article.
- The Impact of Excessive Fructose Intake on Adipose Tissue and the Development of Childhood Obesity. Azevedo-Martins AK, Santos MP, Abayomi J, Ferreira NJR, Evangelista FS. Azevedo-Martins AK, et al. Nutrients. 2024 Mar 25;16(7):939. doi: 10.3390/nu16070939. Nutrients. 2024. PMID: 38612973 Free PMC article. Review.
- NHS Digital. National Statistics: National Child Measurement Programme, England. 2017/18. Accessed April, 2021. https://digital.nhs.uk/data-and-information/publications/statistical/nat...
- Viner RM, Kinra S, Christie D, et al. Improving the assessment and management of obesity in UK children and adolescents: the PROMISE research programme including a RCT. Programme Grants for Applied Research, No. 8.3. 2020.
- Baker C. Briefing paper: obesity statistics. House of Commons Library. 2019;3336:1-20.
- Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(3):709-757.
- Gungor NK. Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol. 2014;6(3):129-143.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Ovid Technologies, Inc.
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Unraveling childhood obesity: a grounded theory approach to psychological, social, parental, and biological factors.

Graphical Abstract
1. Introduction
2. materials and methods, 2.1. methodology, 2.2. inclusion-exclusion criteria, 2.3. search, 2.4. building the grounded theory, 4. discussion, 4.1. social factors, 4.2. biological-genetic factors, 4.3. psychological factors, 4.4. “family condition-related factors”, “parenting style factors”, and “feeding and health related practices”, 4.5. consequences of obesity, 4.6. grounded theory, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest, appendix a. the categories, subcategories, and codes that emerged.
| CATEGORY 1. SOCIAL FACTORS | RELATED WITH PARENTAL SOCIAL STATUS: Socioeconomic status, Low or medium income, Social class, Occupation of the parents, Economic situation of family, Educational level of the parents (particularly maternal education), Unemployment of the parents, Poverty, Social vulnerabilities, Prolonged maternal full-time employment, Parental unemployment (particularly paternal unemployment, Migrant status, Occupational prestige, Poor quality of life, Parental cognitions |
| RELATED WITH SPECIFIC TIME PERIODS: The impact of COVID-19, The impact of the measures for the management of COVID-19, Consumption of cheap and easily available high-calorie food as a lifestyle, Decreased or lack of physical activity as a lifestyle, Lifestyle changes in teenagers, Overconsumption of foods and beverages as a lifestyle, Lack of undertaking physical activity in sport clubs in boys, Change in nutritional habits, Social changes, Generation specific effects, Lifestyle behaviors during pregnancy, Snacking dietary pattern in school children | |
| RELATED WITH SPECIFIC GEOGRAPHIC LOCATIONS AND CULTURES: Living in rural areas, Poor-quality environments, Early feeding practices supported by family culture, Socioeconomic deprivation during the prenatal period and early childhood, Epidemiologic and demographic transitions, Urbanization, Affluence, Political environment, Failing economic environment, Cultural effects, Social inequality as a result of economic insecurity | |
| RELATED WITH SPECIFIC IDEOLOGIES: Cultural beliefs that define a larger infant as representing a healthy and active child, Bogus beliefs and taboos, The concept that “chubby children look cute and lovely”, The concept that “overweight is a minor problem”, The concept that “a large infant is an indication of successful mothering”, Low subjective perceptions of social position, Gender inequalities, Gender roles, Women having primary responsibility in food parenting practices and nutrition, Fathers’ and mother’s beliefs and concerns about nutrition and physical activity, Mistakes of the parents on children’s appropriate diet and weight | |
| RELATED WITH SOCIAL NETWORKS AND OTHER INFLUENCING FACTORS: Lack of support of parents in interventions aimed at the prevention and management of overweight, School environment, Lack of school-based strategies for obesity prevention, Low support from formal and informal sources, Low social support, Minimal social networks, Societal neglect, Lack of guidance of recommended dietary guidelines, Bereavement, Language barrier, Culture shock and lack of acceptance by the new nation in migrant children, Psychosocial stress and feelings of insecurity, Effect of the media, Intergenerational transmission of social disadvantage and health outcomes, Lack of nutritional discipline | |
| CATEGORY 2. GENETIC AND BIOLOGICAL FACTORS | GENETIC FACTORS: Age (greater effects on youngest), Gender (greater genetic effects on boys), Combination of the gender of both parent and child, Child’s birth weight, Familial height and weight, Height, Mother’s age at delivery, The composition of bacteria in the gut, the human microbiome, Genes influencing dopamine and serotonin function, Changes to the precursor stem cell of adipose cells and neurons related to appetite regulation, Epigenetic adaptations and changes, Intergenerational influences, Genetic makeup of individuals, Slow metabolism, Genomics |
| BIOLOGICAL FACTORS: Mechanism of metabolic programming, Heredity, Monogenic or endocrine causes, Metabolic pathways, Hormonal signaling, Altered glucose metabolism, Growth trajectory, Epigenetic influences that cause heritable alterations in gene expression, Intergenerational transfer of obesity, Intrauterine environment and biological programming, Developmental origins of disease, Low fat-free mass, Functional connectivity between the ventral striatum and emotion/motor preparation structures, Connectivity between the ventral striatum and amygdala and attention-related regions, Inflammatory markers, Earlier onset of puberty in females | |
| FACTORS DURING PREGNANCY AND PRENATAL PERIOD: Mother’s diet during pregnancy, Maternal weight gain during pregnancy, Maternal obesity during the first trimester of pregnancy, Excess maternal weight prior to conception, Healthy diet and regular physical activity during pregnancy, Altered metabolism in offspring resulting from variations in the father’s diet, Hormonal signaling during pregnancy, Altered glucose metabolism during pregnancy, Exposure to leptin during the prenatal period, Changes in certain metabolic pathways during pregnancy, Alterations in maternal metabolism, Under- and overnutrition and micronutrient intake during pregnancy, Maternal biology, Gestational diabetes, Greater methylation of specific genes prenatally, Mothers unique influence on offspring body composition, possibly through intrauterine mechanisms, Excessive gestational weight gain (GWG), Rapid infant weight gain, Excess weight at ages 6 months, 1 year, and 2 years, Maternal and parental smoking during the prenatal period, Exercise during pregnancy, The food that a mother consumes and the experiences of taste and smell that function during fetal life, Changes to the placenta, Exercise during pregnancy | |
| BIOLOGICAL AND OTHER INDICATORS FROM THE PARENTS: Abnormal body mass in at least one of the parents, Obesity in both parents affects boys and girls, Obese parents affect sons, Obese mothers affect daughters, Parents slimness in childhood, Parent’s diet, Taste and nutrition preferences of parents, Parents’ smoking habits affect children and especially girls, Paternal and maternal smoking during pregnancy, Mothers’ nutritional status throughout her life, Food cue responsiveness, Maternal smoking during her life | |
| CATEGORY 3. FAMILY CONDITION-RELATED FACTORS | PSYCHO-EMOTIONAL FACTORS RELATED WITH FAMILY AND PARENTS: Stress-coping styles presented by the mothers, Maternal stress, Lack of the ability of parents to regulate their emotions (sadness, stress, etc.), Child maltreatment, Quality of child care, Instrumental feeding, Emotional feeding, The parents’ experience of stress after the birth of the child and during toddlerhood, Insufficient capacity of mothers to decode nonverbal expressions of emotions, Fathers’ mixed levels of self-efficacy in food and activity parenting practices, Resistance from children as a major barrier to promoting healthy eating and physical activity at home |
| FAMILY-MEMBERS RELATIONAL FACTORS: Difficulties in family relationships, Poor family functioning, Home environment factors, Emotional climate during meals, Poor communication, Poor behavior control, High levels of family conflict, Low family hierarchy values, Discord between parents, Violence, Household dysfunction, The role of food in family gatherings, Family cohesion and flexibility, Family food rules or rituals | |
| COGNITIVE PERCEPTIONS AND BEHAVIORAL FACTORS OF THE PARENTS: Taste and nutrition preferences of parents, Mothers’ nutritional status throughout her life, Parental healthy modeling, Low parental concerns about their child’s thinness, Parental concern about child weight, Parental difficulty in recognizing weight problems, Parental perceptions of the diet, Authoritative feeding style, Authoritarian (restrictive) feeding style, Autonomy-supportive food parenting practices | |
| PREVAILING FAMILY CONDITIONS: Having only one son in the family, Parental separation or divorce, Living with a substance abuser, Imprisonment of a household member, Witnessing a parent being abused, Living with a mentally ill person, The effect of birth order, Being part of nontraditional families, Number of children in family, Adverse experiences in childhood, Limited time to take care of children | |
| CATEGORY 4. PSYCHOLOGICAL FACTORS | MENTAL HEALTH ISSUES: Depression, Anxiety, Eating disorders, Coping with stress, Infant’s temperament, Autism spectrum disorders, Attention-deficit hyperactivity disorder, Alexithymia, Behavior disorders, Negative emotionality, Negative self-evaluation, Poor self-image, Body dissatisfaction, Conduct problems, Hyperkinetic disorders (hyperactivity, inattention, and impulsivity), Peer relationship problems and prosocial behavior, Coping with stressful situations, Coping with traumatic experiences |
| PSYCHOLOGICAL FACTORS CONNECTED WITH FOOD CONSUMPTION: Emotion regulation with food, Disturbing behavior, Neophobia (fear of new foods), Food addiction, Tantrums over food, Delay of gratification, Overeating amongst girls, Binge eating, Emotional feeding from parents, Inability to monitor food intake, Emotional eating, Eating in the absence of hunger, Higher food responsiveness (being attracted to food and eating) | |
| COPING WITH EMOTIONS ISSUES: Psychological control, Behavioral regulation, Social-emotional competence, Emotion and self-regulation, Inhibitory control, Emotional reactivity, Increased levels of negative affect, Less emotional awareness, Difficulty in coping with negative emotions, Child emotional insecurity, Problems with experiencing, describing, and identifying one’s emotions, Internalizing or externalizing difficulties, Emotional abuse | |
| CATEGORY 5. PARENTING STYLE | GENERAL PARENTING STYLE: Strict parenting style, Authoritative parenting style (Balanced use of open, communicative warmth and assertive discipline), Permissive parenting style (little to no discipline or control over a child), Authoritarian parenting style (Heavy use of control and discipline with little warm communication), Neglectful parenting style, Responsiveness of the parent, Demandingness of the parent (especially of the mother), Uninvolved parenting style, Negative parental practices, Uninvolved parenting style (parents who are low on both warmth and control), Inconsistent parenting, Poor parenting |
| RELATED TO EMOTIONAL AND PSYCHOLOGICAL SITUATIONS: Monitoring and controlling child activities and deviant behaviors, Lack of praise, Levels of parental and maternal emotional warmth, Parental psychological control, Family communication, Negative paternal and maternal communication, Parental neglect, Insecure attachment relationship, Lack of acceptance from the parents, Poor mother–child relationship followed by an insecure mother–child attachment, Parental interpersonal dysphoria, Maternal intrusiveness, Levels of parental support and encouragement, Overprotection, Coercive control, Differential parental treatment to the kids of a family, Soothing strategies for infant/toddler distress and fussiness, Parental responsiveness to their child’s needs, Absent parents, Maternal depression, self-esteem, financial strain, and maternal distress | |
| CATEGORY 6. FEEDING AND HEALTH RELATED PRACTICES | PRACTICES AROUND FOOD CONSUMPTION: Eating habits such as not drinking enough water, or not chewing food adequately, Not offering assistance during mealtimes, Early introduction of complementary solid foods, Exposure to a certain food type after a period of restriction to it, Pressing the children to eat, Not promoting self-regulation of the children, Parental strict limitations in food, Food fussiness, Absence of frequent family meals, Formula-fed infants, Age-inappropriate feeding, Greater role for fat and added sugars in foods, Reduced intakes of complex carbohydrates and dietary fiber, Reduced fruit and vegetable intake, Eating rate, Disinhibited eating, Use of food as a reward, Large portions, Response to children’s hunger and fullness cues, Breastfeeding period |
| HEALTH RELATED PRACTICES: Not enhancing physical activity, Not controlling screen time, Absence of establishment of rules for sleep schedules, Absence of age-appropriate sleep patterns and duration, Enhancing sedentary behavior, Use of car seats and strollers, Exposure to television and media, Sleep deprivation, Having a television in children’s bedrooms, Quality of sleep, Medication, Having the television on during dinner, Leisure time activities, Drug, alcohol, cigarette consumption, Not doing things together with children, Spending time with children in physical activities | |
| PRACTICES AROUND FOOD PREPARATION AND AVAILABILITY: Availability of healthy food at home, Not educating children about nutrition, No involvement of the children in preparing meals, Not offering different choices for food consumption, Not discussing food choices with children, Absence of flexible, individualized dietary plan, Absence of clear and consistent rules related to food, Not respecting infant’s or toddler’s flavor or food preferences, Not respecting appetitive characteristics and traits, Allowing children unrestricted access to inappropriate foods or displaying no supportive guidance, Asserting strict control over all feeding behaviors, Not enhancing the children to eat both new and familiar foods, Intake of unhealthy snack foods as an easy choice | |
| CATEGORY 7. CONSEQUENCES OF OBESITY | SOCIAL: Weigh related stigma, Body image concerns, Being avoided, ignored, or the subject of negative rumors, Problems of integration with peers, Bullying, Joint problems, Dissatisfaction with one’s own body, High school drop-out, Reduced work integration, Poor quality of life |
| PSYCHOLOGICAL: Emotional difficulty, Mental disorders, Higher rates of sadness, loneliness, and nervousness, Decreased self-esteem, Psychological problems, Poor self-image, Depression, Anxiety, Psychiatric health problems, Suicidality, Poorer well-being | |
| BIOLOGICAL: Increases mortality, Sixth risk factor for death, Cardiovascular disorders, Metabolic disorders, Adult obesity, Diabetes and insulin resistance, Renal and liver disorders, Musculoskeletal disorders, Respiratory disorders, Neurological disorders, Chronic diseases, Menstrual disorders, Fertility challenges, Cancers of the esophagus, pancreas, colon and rectum, breast (post-menopausal), endometrium, and kidney, Lower physical functioning performance, High blood pressure, Asthma |
- Rolland-Cachera, M.F.; Sempé, M.; Guilloud-Bataille, M.; Patois, E.; Péquignot-Guggenbuhl, F.; Fautrad, V. Adiposity Indices in Children. Am. J. Clin. Nutr. 1982 , 36 , 178–184. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kêkê, L.M.; Samouda, H.; Jacobs, J.; di Pompeo, C.; Lemdani, M.; Hubert, H.; Zitouni, D.; Guinhouya, B.C. Body Mass Index and Childhood Obesity Classification Systems: A Comparison of the French, International Obesity Task Force (IOTF) and World Health Organization (WHO) References. Rev. Epidemiol. Sante Publique 2015 , 63 , 173–182. [ Google Scholar ] [ CrossRef ]
- Kamal, S.A. In search of a definition of childhood obesity. Int. J. Biol. Biotechnol. 2017 , 14 , 49–67. [ Google Scholar ]
- Faienza, M.F.; Chiarito, M.; Molina-Molina, E.; Shanmugam, H.; Lammert, F.; Krawczyk, M.; D’Amato, G.; Portincasa, P. Childhood Obesity, Cardiovascular and Liver Health: A Growing Epidemic with Age. World J. Pediatr. 2020 , 16 , 438–445. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide Epidemic of Obesity. In Obesity and Obstetrics , 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. ISBN 978-0-12-817921-5. [ Google Scholar ]
- Frazier, J.A.; Li, X.; Kong, X.; Hooper, S.R.; Joseph, R.M.; Cochran, D.M.; Kim, S.; Fry, R.C.; Brennan, P.A.; Msall, M.E.; et al. Perinatal Factors and Emotional, Cognitive, and Behavioral Dysregulation in Childhood and Adolescence. J. Am. Acad. Child. Adolesc. Psychiatry 2023 , 62 , 1351–1362. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vazquez, C.E.; Cubbin, C. Socioeconomic Status and Childhood Obesity: A Review of Literature from the Past Decade to Inform Intervention Research. Curr. Obes. Rep. 2020 , 9 , 562–570. [ Google Scholar ] [ CrossRef ]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022 , 10 , 351–365. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Omer, T.A.M. The Causes of Obesity: An in-Depth Review. Adv. Obes. Weight Manag. Control 2020 , 10 , 90–94. [ Google Scholar ] [ CrossRef ]
- Chatham, R.E.; Mixer, S.J. Cultural Influences on Childhood Obesity in Ethnic Minorities: A Qualitative Systematic Review. J. Transcult. Nurs. 2020 , 31 , 87–99. [ Google Scholar ] [ CrossRef ]
- Hemmingsson, E.; Nowicka, P.; Ulijaszek, S.; Sørensen, T.I.A. The Social Origins of Obesity within and across Generations. Obes. Rev. 2023 , 24 , e13514. [ Google Scholar ] [ CrossRef ]
- Deal, B.J.; Huffman, M.D.; Binns, H.; Stone, N.J. Perspective: Childhood Obesity Requires New Strategies for Prevention. Adv. Nutr. 2020 , 11 , 1071–1078. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kansra, A.R.; Lakkunarajah, S.; Jay, M.S. Childhood and Adolescent Obesity: A Review. Front. Pediatr. 2020 , 8 , 581461. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marcus, C.; Danielsson, P.; Hagman, E. Pediatric Obesity-Long-Term Consequences and Effect of Weight Loss. J. Intern. Med. 2022 , 292 , 870–891. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Handakas, E.; Lau, C.H.; Alfano, R.; Chatzi, V.L.; Plusquin, M.; Vineis, P.; Robinson, O. A Systematic Review of Metabolomic Studies of Childhood Obesity: State of the Evidence for Metabolic Determinants and Consequences. Obes. Rev. 2022 , 23 (Suppl. S1), e13384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Caprio, S.; Santoro, N.; Weiss, R. Childhood Obesity and the Associated Rise in Cardiometabolic Complications. Nat. Metab. 2020 , 2 , 223–232. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rojo, M.; Solano, S.; Lacruz, T.; Baile, J.I.; Blanco, M.; Graell, M.; Sepúlveda, A.R. Linking Psychosocial Stress Events, Psychological Disorders and Childhood Obesity. Children 2021 , 8 , 211. [ Google Scholar ] [ CrossRef ]
- Thaker, V.V.; Osganian, S.K.; deFerranti, S.D.; Sonneville, K.R.; Cheng, J.K.; Feldman, H.A.; Richmond, T.K. Psychosocial, Behavioral and Clinical Correlates of Children with Overweight and Obesity. BMC Pediatr. 2020 , 20 , 291. [ Google Scholar ] [ CrossRef ]
- Smith, J.D.; Fu, E.; Kobayashi, M.A. Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu. Rev. Clin. Psychol. 2020 , 16 , 351–378. [ Google Scholar ] [ CrossRef ]
- Campos, P.; Luceño, L.; Aguirre, C. Physical Spaces in Higher Education as Scenarios of Learning Innovation: Compositional and Formative Synergies among Architecture, Music, and Fashion. Eur. J. Investig. Health Psychol. Educ. 2021 , 11 , 1166–1180. [ Google Scholar ] [ CrossRef ]
- Snelling, A.; Hawkins, M.; McClave, R.; Irvine Belson, S. The Role of Teachers in Addressing Childhood Obesity: A School-Based Approach. Nutrients 2023 , 15 , 3981. [ Google Scholar ] [ CrossRef ]
- Spinelli, A.; Censi, L.; Mandolini, D.; Ciardullo, S.; Salvatore, M.A.; Mazzarella, G.; Nardone, P.; 2019 OKkio alla SALUTE Group. Inequalities in Childhood Nutrition, Physical Activity, Sedentary Behaviour and Obesity in Italy. Nutrients 2023 , 15 , 3893. [ Google Scholar ] [ CrossRef ]
- Buksh, S.M.; Hay, P.; de Wit, J.B.F. Perceptions on Healthy Eating Impact the Home Food Environment: A Qualitative Exploration of Perceptions of Indigenous Food Gatekeepers in Urban Fiji. Nutrients 2023 , 15 , 3875. [ Google Scholar ] [ CrossRef ]
- Figueira, M.; Araújo, J.; Gregório, M.J. Monitoring Food Marketing Directed to Portuguese Children Broadcasted on Television. Nutrients 2023 , 15 , 3800. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bondyra-Wiśniewska, B.; Harton, A. Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data. Nutrients 2023 , 15 , 3646. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gioxari, A.; Amerikanou, C.; Peraki, S.; Kaliora, A.C.; Skouroliakou, M. Eating Behavior and Factors of Metabolic Health in Primary Schoolchildren: A Cross-Sectional Study in Greek Children. Nutrients 2023 , 15 , 3592. [ Google Scholar ] [ CrossRef ]
- Pavlidou, E.; Papandreou, D.; Taha, Z.; Mantzorou, M.; Tyrovolas, S.; Kiortsis, D.N.; Psara, E.; Papadopoulou, S.K.; Yfantis, M.; Spanoudaki, M.; et al. Association of Maternal Pre-Pregnancy Overweight and Obesity with Childhood Anthropometric Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study. Nutrients 2023 , 15 , 3384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Silva-Uribe, M.; Máynez-López, F.; Denova-Gutiérrez, E.; Muñoz-Guerrero, B.; Omaña-Guzmán, I.; Messiah, S.E.; Ruíz-Arroyo, A.; Lozano-González, E.; Villanueva-Ortega, E.; Muñoz-Aguirre, P.; et al. Validation of the Childhood Family Mealtime Questionnaire in Mexican Adolescents with Obesity and Their Caregivers. Nutrients 2023 , 15 , 4937. [ Google Scholar ] [ CrossRef ]
- Calcaterra, V.; Rossi, V.; Tagi, V.M.; Baldassarre, P.; Grazi, R.; Taranto, S.; Zuccotti, G. Food Intake and Sleep Disorders in Children and Adolescents with Obesity. Nutrients 2023 , 15 , 4736. [ Google Scholar ] [ CrossRef ]
- Mannino, A.; Sarapis, K.; Mourouti, N.; Karaglani, E.; Anastasiou, C.A.; Manios, Y.; Moschonis, G. The Association of Maternal Weight Status throughout the Life-Course with the Development of Childhood Obesity: A Secondary Analysis of the Healthy Growth Study Data. Nutrients 2023 , 15 , 4602. [ Google Scholar ] [ CrossRef ]
- Lin, S.-F.; Zive, M.M.; Schmied, E.; Helm, J.; Ayala, G.X. The Effects of a Multisector, Multilevel Intervention on Child Dietary Intake: California Childhood Obesity Research Demonstration Study. Nutrients 2023 , 15 , 4449. [ Google Scholar ] [ CrossRef ]
- Blake, M.K.; Ma, R.; Cardenas, E.V.; Varanloo, P.; Agosto, Y.; Velasquez, C.; Espina, K.A.; Palenzuela, J.; Messiah, S.E.; Natale, R.A. Infant Nutrition and Other Early Life Risk Factors for Childhood Obesity According to Disability Status. Nutrients 2023 , 15 , 4394. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arellano-Alvarez, P.; Muñoz-Guerrero, B.; Ruiz-Barranco, A.; Garibay-Nieto, N.; Hernandez-Lopez, A.M.; Aguilar-Cuarto, K.; Pedraza-Escudero, K.; Fuentes-Corona, Z.; Villanueva-Ortega, E. Barriers in the Management of Obesity in Mexican Children and Adolescents through the COVID-19 Lockdown—Lessons Learned and Perspectives for the Future. Nutrients 2023 , 15 , 4238. [ Google Scholar ] [ CrossRef ]
- González-Torres, M.L.; Garza-Olivares, X.; Navarro-Contreras, G.; González-Orozco, L.A. Validation of the Scale on Parental Feeding Behaviors (ECOPAL) for Caregivers of Mexican Children. Nutrients 2023 , 15 , 3698. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Borkhoff, S.A.; Parkin, P.C.; Birken, C.S.; Maguire, J.L.; Macarthur, C.; Borkhoff, C.M. Examining the Double Burden of Underweight, Overweight/Obesity and Iron Deficiency among Young Children in a Canadian Primary Care Setting. Nutrients 2023 , 15 , 3635. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, J.H.; Lee, E.; Ha, E.K.; Lee, G.C.; Shin, J.; Baek, H.-S.; Choi, S.-H.; Shin, Y.H.; Han, M.Y. Infant Feeding Pattern Clusters Are Associated with Childhood Health Outcomes. Nutrients 2023 , 15 , 3065. [ Google Scholar ] [ CrossRef ]
- Strauss, A.; Corbin, J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory , 2nd ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1998; ISBN 978-0-8039-5939-2. [ Google Scholar ]
- Glaser, B.G.; Strauss, A.L. The Discovery of Grounded Theory: Strategies for Qualitative Research , 1st ed.; Routledge: London, UK, 2017; ISBN 978-0-203-79320-6. [ Google Scholar ]
- Bidopia, T. The Development of Disordered Eating and Body Image Issues in Latina Adolescent Girls: A Grounded Theory Approach. Master’s Thesis, Fordham University, New York, NY, USA, 2023; pp. 1–110. [ Google Scholar ]
- Vaezghasemi, M.; Öhman, A.; Ng, N.; Hakimi, M.; Eriksson, M. Concerned and Conscious, but Defenceless-the Intersection of Gender and Generation in Child Malnutrition in Indonesia: A Qualitative Grounded Theory Study. Glob. Health Action 2020 , 13 , 1744214. [ Google Scholar ] [ CrossRef ]
- Nguyen, T.; Trat, T.; Tieu, N.T.; Vu, L.; Sokal-Gutierrez, K. Key Informants’ Perspectives on Childhood Obesity in Vietnam: A Qualitative Study. Matern. Child Health J. 2022 , 26 , 1811. [ Google Scholar ] [ CrossRef ]
- Verga, S.M.P.; de Azevedo Mazza, V.; Teodoro, F.C.; Girardon-Perlini, N.M.O.; Marcon, S.S.; de Almeida Fernandes Rodrigues, É.T.; Ruthes, V.B.T.N.M. The Family System Seeking to Transform Its Eating Behavior in the Face of Childhood Obesity. Rev. Bras. Enferm. 2022 , 75 , e20210616. [ Google Scholar ] [ CrossRef ]
- Jansen, E.; Harris, H.; Rossi, T. Fathers’ Perceptions of Their Role in Family Mealtimes: A Grounded Theory Study. J. Nutr. Educ. Behav. 2020 , 52 , 45–54. [ Google Scholar ] [ CrossRef ]
- Ayre, S.K.; White, M.J.; Harris, H.A.; Byrne, R.A. “I’m Having Jelly Because You’ve Been Bad!”: A Grounded Theory Study of Mealtimes with Siblings in Australian Families. Matern. Child Nutr. 2023 , 19 , e13484. [ Google Scholar ] [ CrossRef ]
- Middleton, G.; Golley, R.K.; Patterson, K.A.; Coveney, J. Barriers and Enablers to the Family Meal across Time; a Grounded Theory Study Comparing South Australian Parents’ Perspectives. Appetite 2023 , 191 , 107091. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Eaton, E. Parental Perspectives of the Barriers to Sustaining Health Behaviour Change for Their Child Living with Overweight or Obesity–A Grounded Theory Study. Ph.D. Thesis, University of Essex, Colchester, UK, 2023. [ Google Scholar ]
- Bisogni, C.A.; Jastran, M.; Seligson, M.; Thompson, A. How People Interpret Healthy Eating: Contributions of Qualitative Research. J. Nutr. Educ. Behav. 2012 , 44 , 282–301. [ Google Scholar ] [ CrossRef ]
- Ponterotto, J.G. Qualitative Research in Counseling Psychology: A Primer on Research Paradigms and Philosophy of Science. J. Couns. Psychol. 2005 , 52 , 126–136. [ Google Scholar ] [ CrossRef ]
- Strauss, A.; Corbin, J. Basics of Qualitative Research Techniques , 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 1998. [ Google Scholar ]
- Corbin, J.; Strauss, A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory , 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2008; ISBN 978-1-4522-3015-3. [ Google Scholar ]
- Charmaz, K. Grounded Theory: Main Characteristics. Qual. Anal. Eight Approaches Soc. Sci. 2020 , 1 , 195–222. [ Google Scholar ]
- Mohajan, D.; Mohajan, H. Classic Grounded Theory: A Qualitative Research on Human Behavior. Stud. Soc. Sci. Humanit. 2022 , 2 , 1–7. [ Google Scholar ] [ CrossRef ]
- Ligita, T.; Harvey, N.; Wicking, K.; Nurjannah, I.; Francis, K. A Practical Example of Using Theoretical Sampling throughout a Grounded Theory Study: A Methodological Paper. Qual. Res. J. 2020 , 20 , 116–126. [ Google Scholar ] [ CrossRef ]
- Coleman, P. Validity and Reliability within Qualitative Research for the Caring Sciences. Int. J. Caring Sci. 2022 , 14 , 2041–2045. [ Google Scholar ]
- Morse, J.M.; Barrett, M.; Mayan, M.; Olson, K.; Spiers, J. Verification Strategies for Establishing Reliability and Validity in Qualitative Research. Int. J. Qual. Methods 2002 , 1 , 13–22. [ Google Scholar ] [ CrossRef ]
- Hendrawan, E.; Meisel, M.; Sari, D.N. Analysis and implementation of computer network systems using software draw.io. Asia Inf. Syst. J. 2023 , 2 , 9–15. [ Google Scholar ] [ CrossRef ]
- Glaser, B.G. Doing Grounded Theory: Issues and Discussions ; Sociology Press: Mill Valley, CA, USA, 1998; ISBN 978-1-884156-11-3. [ Google Scholar ]
- Thornberg, R. Informed Grounded Theory. Scand. J. Educ. Res. 2012 , 56 , 243–259. [ Google Scholar ] [ CrossRef ]
- Thornberg, R.; Charmaz, K. The SAGE Handbook of Qualitative Data Analysis ; SAGE Publications Ltd.: Thousand Oaks, CA, USA, 2014; ISBN 978-1-4462-8224-3. [ Google Scholar ]
- Batko, B.; Kowal, M.; Szwajca, M.; Pilecki, M. Relationship between biopsychosocial factors, body mass and body composition in preschool children. Psychiatr. I Psychol. Klin.-J. Psychiatry Clin. Psychol. 2020 , 20 , 164–173. [ Google Scholar ] [ CrossRef ]
- Carnell, S.; Kim, Y.; Pryor, K. Fat Brains, Greedy Genes, and Parent Power: A Biobehavioural Risk Model of Child and Adult Obesity. Int. Rev. Psychiatry 2012 , 24 , 189–199. [ Google Scholar ] [ CrossRef ]
- Chatzidaki, E.; Chioti, V.; Mourtou, L.; Papavasileiou, G.; Kitani, R.-A.; Kalafatis, E.; Mitsis, K.; Athanasiou, M.; Zarkogianni, K.; Nikita, K. Parenting Styles and Psychosocial Factors of Mother–Child Dyads Participating in the ENDORSE Digital Weight Management Program for Children and Adolescents during the COVID-19 Pandemic. Children 2024 , 11 , 107. [ Google Scholar ] [ CrossRef ]
- Coleman, J.R.I.; Krapohl, E.; Eley, T.C.; Breen, G. Individual and Shared Effects of Social Environment and Polygenic Risk Scores on Adolescent Body Mass Index. Sci. Rep. 2018 , 8 , 6344. [ Google Scholar ] [ CrossRef ]
- Do, L.M.; Larsson, V.; Tran, T.K.; Nguyen, H.T.; Eriksson, B.; Ascher, H. Vietnamese Mother’s Conceptions of Childhood Overweight: Findings from a Qualitative Study. Glob. Health Action 2016 , 9 , 30215. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Faith, M.S.; Berkowitz, R.I.; Stallings, V.A.; Kerns, J.; Storey, M.; Stunkard, A.J. Eating in the Absence of Hunger: A Genetic Marker for Childhood Obesity in Prepubertal Boys? Obesity 2006 , 14 , 131–138. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Haire-Joshu, D.; Tabak, R. Preventing Obesity Across Generations: Evidence for Early Life Intervention. Annu. Rev. Public Health 2016 , 37 , 253–271. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Holmen, T.L.; Bratberg, G.; Krokstad, S.; Langhammer, A.; Hveem, K.; Midthjell, K.; Heggland, J.; Holmen, J. Cohort Profile of the Young-HUNT Study, Norway: A Population-Based Study of Adolescents. Int. J. Epidemiol. 2014 , 43 , 536–544. [ Google Scholar ] [ CrossRef ]
- Iguacel, I.; Fernández-Alvira, J.M.; Ahrens, W.; Bammann, K.; Gwozdz, W.; Lissner, L.; Michels, N.; Reisch, L.; Russo, P.; Szommer, A.; et al. Prospective Associations between Social Vulnerabilities and Children’s Weight Status. Results from the IDEFICS Study. Int. J. Obes. 2018 , 42 , 1691–1703. [ Google Scholar ] [ CrossRef ]
- Ji, M.; An, R. Parenting styles in relation to childhood obesity, smoking and drinking: A gene–environment interaction study. J. Hum. Nutr. 2022 , 35 , 625–633. [ Google Scholar ] [ CrossRef ]
- Regber, S.; Dahlgren, J.; Janson, S. Neglected Children with Severe Obesity Have a Right to Health: Is Foster Home an Alternative?—A Qualitative Study. Child Abus. Negl. 2018 , 83 , 106–119. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ji, M.; An, R. Parental Effects on Obesity, Smoking, and Drinking in Children and Adolescents: A Twin Study. J. Adolesc. Health 2022 , 71 , 196–203. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Paul, I.M.; Williams, J.S.; Anzman-Frasca, S.; Beiler, J.S.; Makova, K.D.; Marini, M.E.; Hess, L.B.; Rzucidlo, S.E.; Verdiglione, N.; Mindell, J.A. The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) Study. BMC Pediatr. 2014 , 14 , 184. [ Google Scholar ] [ CrossRef ]
- Van De Beek, C.; Hoek, A.; Painter, R.C.; Gemke, R.J.; Van Poppel, M.N.; Geelen, A.; Groen, H.; Mol, B.W.; Roseboom, T.J. Women, their Offspring and improving lifestyle for Better cardiovascular health of both (WOMB project): A protocol of the follow-up of a multicenter randomized controlled trial. BMJ Open 2018 , 8 , e016579. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vedanthan, R.; Bansilal, S.; Soto, A.V.; Kovacic, J.C.; Latina, J.; Jaslow, R.; Santana, M.; Gorga, E.; Kasarskis, A.; Hajjar, R.; et al. Family-Based Approaches to Cardiovascular Health Promotion. J. Am. Coll. Cardiol. 2016 , 67 , 1725–1737. [ Google Scholar ] [ CrossRef ]
- Murrin, C.M.; Kelly, G.E.; Tremblay, R.E.; Kelleher, C.C. Body Mass Index and Height over Three Generations: Evidence from the Lifeways Cross-Generational Cohort Study. BMC Public Health 2012 , 12 , 81. [ Google Scholar ] [ CrossRef ]
- Oparaocha, E. Childhood Obesity in Nigeria: Causes and Suggestions for Control. Niger. J. Parasitol. 2018 , 39 , 1–7. [ Google Scholar ] [ CrossRef ]
- Kiefner-Burmeister, A.; Hinman, N. The Role of General Parenting Style in Child Diet and Obesity Risk. Curr. Nutr. Rep. 2020 , 9 , 14–30. [ Google Scholar ] [ CrossRef ]
- Poulain, T.; Baber, R.; Vogel, M.; Pietzner, D.; Kirsten, T.; Jurkutat, A.; Hiemisch, A.; Hilbert, A.; Kratzsch, J.; Thiery, J. The LIFE Child Study: A Population-Based Perinatal and Pediatric Cohort in Germany. Eur. J. Epidemiol. 2017 , 32 , 145–158. [ Google Scholar ] [ CrossRef ]
- McDonald, G.; Faga, P.; Jackson, D.; Mannix, J.; Firtko, A. Mothers’ Perceptions of Overweight and Obesity in Their Children. Aust. J. Adv. Nurs. 2005 , 23 , 8–13. [ Google Scholar ]
- Zhang, Y.; Hurtado, G.A.; Flores, R.; Alba-Meraz, A.; Reicks, M. Latino Fathers’ Perspectives and Parenting Practices Regarding Eating, Physical Activity, and Screen Time Behaviors of Early Adolescent Children: Focus Group Findings. J. Acad. Nutr. Diet 2018 , 118 , 2070–2080. [ Google Scholar ] [ CrossRef ]
- Suder, A.; Chrzanowska, M. Risk factors for abdominal obesity in children and adolescents from Cracow, Poland (1983–2000). J. Biosoc. Sci. 2015 , 47 , 203–219. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Russell, C.G.; Russell, A. Biological and Psychosocial Processes in the Development of Children’s Appetitive Traits: Insights from Developmental Theory and Research. Nutrients 2018 , 10 , 692. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mazzeo, S.; Mitchell, K.; Gerke, C.; Bulik, C. Parental Feeding Style and Eating Attitudes: Influences on Children’s Eating Behavior. Curr. Nutr. Food Sci. 2006 , 2 , 275–295. [ Google Scholar ] [ CrossRef ]
- Levitt, H.M. Qualitative Generalization, Not to the Population but to the Phenomenon: Reconceptualizing Variation in Qualitative Research. Qual. Psychol. 2021 , 8 , 95. [ Google Scholar ] [ CrossRef ]
- Iguacel, I.; Gasch-Gallén, Á.; Ayala-Marín, A.M.; De Miguel-Etayo, P.; Moreno, L.A. Social Vulnerabilities as Risk Factor of Childhood Obesity Development and Their Role in Prevention Programs. Int. J. Obes. 2021 , 45 , 1–11. [ Google Scholar ] [ CrossRef ]
- Grube, M.; Bergmann, S.; Keitel, A.; Herfurth-Majstorovic, K.; Wendt, V.; von Klitzing, K.; Klein, A.M. Obese Parents–Obese Children? Psychological-Psychiatric Risk Factors of Parental Behavior and Experience for the Development of Obesity in Children Aged 0–3: Study Protocol. BMC Public Health 2013 , 13 , 1193. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Polit, D.F.; Beck, C.T. Generalization in Quantitative and Qualitative Research: Myths and Strategies. Int. J. Nurs. Stud. 2010 , 47 , 1451–1458. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Author-Authors | Year | Title | Types of Factors | Reference Number |
|---|---|---|---|---|
| Batko, B., Kowal, M., Szwajca, M., and Pilecki, M. | 2020 | Relationship between biopsychosocial factors, body mass and body composition in preschool children | Biological and psychological factors | [ ] |
| Carnell, S., Kim, Y., and Pryor, K. | 2012 | Fat brains, greedy genes, and parent power: A biobehavioral risk model of child and adult obesity | Parental and biological factors | [ ] |
| Chatzidaki, E., Chioti, V., Mourtou, L., Papavasileiou, G., Kitani, R.-A., Kalafatis, E., Mitsis, K., Athanasiou, M., Zarkogianni, K., and Nikita, K. | 2024 | Parenting styles and psychosocial factors of mother–child dyads participating in the ENDORSE digital weight management program for children and adolescents during the COVID-19 pandemic | Parental, social and psychological factors | [ ] |
| Coleman, J. R., Krapohl, E., Eley, T. C., and Breen, G. | 2018 | Individual and shared effects of social environment and polygenic risk scores on adolescent body mass index | Social and biological factors | [ ] |
| Do, L. M., Larsson, V., Tran, T. K., Nguyen, H. T., Eriksson, B., and Ascher, H. | 2016 | Vietnamese mother’s conceptions of childhood overweight: Findings from a qualitative study | Parental factors | [ ] |
| Faith, M. S., Berkowitz, R. I., Stallings, V. A., Kerns, J., Storey, M., and Stunkard, A. J. | 2006 | Eating in the absence of hunger: A genetic marker for childhood obesity in prepubertal boys? | Social factors | [ ] |
| Haire-Joshu, D., and Tabak, R. | 2016 | Preventing obesity across generations: Evidence for early life intervention | Social and biological factors | [ ] |
| Holmen, T. L., Bratberg, G., Krokstad, S., Langhammer, A., Hveem, K., Midthjell, K., Heggland, J., and Holmen, J. | 2014 | Cohort profile of the young-HUNT study, Norway: A population-based study of adolescents | Biological and psychological factors | [ ] |
| Iguacel, I., Fernández-Alvira, J. M., Ahrens, W., Bammann, K., Gwozdz, W., Lissner, L., Michels, N., Reisch, L., Russo, P., and Szommer, A. | 2018 | Prospective associations between social vulnerabilities and children’s weight status. Results from the IDEFICS study | Social factors | [ ] |
| Ji, M. and An, R. | 2022a | Parental effects on obesity, smoking, and drinking in children and adolescents: A twin study | Parental factors | [ ] |
| Ji, M. and An, R. | 2022b | Parenting styles in relation to childhood obesity, smoking, and drinking: A gene–environment interaction study | Social and biological factors | [ ] |
| Kiefner-Burmeister, A., and Hinman, N. | 2020 | The role of general parenting style in child diet and obesity risk | Parental factors | [ ] |
| Grube, M., Bergmann, S., Keitel, A., Herfurth-Majstorovic, K., Wendt, V., von Klitzing, K., and Klein, A.M. | 2013 | Obese parents—obese children? Psychological-psychiatric risk factors of parental behavior and experience for the development of obesity in children aged 0–3: Study protocol | Parental and psychological factors | [ ] |
| Mazzeo, S. E., Mitchell, K. S., Gerke, C. K., and Bulik, C. M. | 2006 | Parental feeding style and eating attitudes: Influences on children’s eating behavior | Parental and psychological factors | [ ] |
| McDonald, G., Faga, P., Jackson, D., Mannix, J., and Firtko, A. | 2005 | Mothers’ perceptions of overweight and obesity in their children | Parental factors | [ ] |
| Murrin, C. M., Kelly, G. E., Tremblay, R. E., and Kelleher, C. C. | 2012 | Body mass index and height over three generations: evidence from the Lifeways cross-generational cohort study | Biological factors | [ ] |
| Oparaocha, E. | 2018 | Childhood obesity in Nigeria: Causes and suggestions for control | Social factors | [ ] |
| Paul, I. M., Williams, J. S., Anzman-Frasca, S., Beiler, J. S., Makova, K. D., Marini, M. E., Hess, L. B., Rzucidlo, S. E., Verdiglione, N., and Mindell, J. A. | 2014 | The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study | Biological factors | [ ] |
| Poulain, T., Baber, R., Vogel, M., Pietzner, D., Kirsten, T., Jurkutat, A., Hiemisch, A., Hilbert, A., Kratzsch, J., and Thiery, J. | 2017 | The LIFE Child study: a population-based perinatal and pediatric cohort in Germany | Biological factors | [ ] |
| Regber, S., Dahlgren, J., and Janson, S. | 2018 | Neglected children with severe obesity have a right to health: Is foster home an alternative?—A qualitative study | Social and parental factors | [ ] |
| Russell, C. G., and Russell, A. | 2018 | Biological and psychosocial processes in the development of children’s appetitive traits: Insights from developmental theory and research | Biological, social and psychological factors | [ ] |
| Suder, A., and Chrzanowska, M. | 2015 | Risk factors for abdominal obesity in children and adolescents from Cracow, Poland (1983–2000) | Biological, social and psychological factors | [ ] |
| Van De Beek, C., Hoek, A., Painter, R. C., Gemke, R. J., Van Poppel, M. N., Geelen, A., Groen, H., Mol, B. W., and Roseboom, T. J. | 2018 | Women, their offspring and improving lifestyle for better cardiovascular health of both (WOMB project): A protocol of the follow-up of a multicenter randomized controlled trial | Biological, social and parental factors | [ ] |
| Vedanthan, R., Bansilal, S., Soto, A. V., Kovacic, J. C., Latina, J., Jaslow, R., Santana, M., Gorga, E., Kasarskis, A., and Hajjar, R. | 2016 | Family-based approaches to cardiovascular health promotion | Biological and parental factors | [ ] |
| Zhang, Y., Hurtado, G. A., Flores, R., Alba-Meraz, A., and Reicks, M. | 2018 | Latino fathers’ perspectives and parenting practices regarding eating, physical activity, and screen time behaviors of early adolescent children: Focus group findings | Parental factors | [ ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Karakitsiou, G.; Plakias, S.; Christidi, F.; Tsiakiri, A. Unraveling Childhood Obesity: A Grounded Theory Approach to Psychological, Social, Parental, and Biological Factors. Children 2024 , 11 , 1048. https://doi.org/10.3390/children11091048
Karakitsiou G, Plakias S, Christidi F, Tsiakiri A. Unraveling Childhood Obesity: A Grounded Theory Approach to Psychological, Social, Parental, and Biological Factors. Children . 2024; 11(9):1048. https://doi.org/10.3390/children11091048
Karakitsiou, Georgia, Spyridon Plakias, Foteini Christidi, and Anna Tsiakiri. 2024. "Unraveling Childhood Obesity: A Grounded Theory Approach to Psychological, Social, Parental, and Biological Factors" Children 11, no. 9: 1048. https://doi.org/10.3390/children11091048
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Preventing Childhood Obesity
- Health Care Strategies
- About Obesity
- What Can Be Done
- Obesity Data and Statistics
Related Topics:
- View All Home
- About Healthy Weight and Growth
- Body Mass Index (BMI)
- About Nutrition
- About Physical Activity
Childhood Obesity Facts
At a glance.
- Approximately 1 in 5 U.S. children and adolescents have obesity.
- Obesity affects some groups more than others, including adolescents, Hispanic and non-Hispanic Black children, and children in families with lower incomes.
- Health care for obesity is expensive for patients and the health care system.
Many U.S. children have obesity
From 2017 to March 2020, the prevalence of obesity among U.S. children and adolescents was 19.7% 1 . This means that approximately 14.7 million U.S. youths aged 2–19 years have obesity.
For children, obesity is defined as having a body mass index (BMI) at or above the 95th percentile for age and sex.
Obesity affects some groups more than others
The prevalence of obesity increased with age. From 2017 to March 2020, obesity prevalence was 12.7% among U.S. children 2–5 years old, 20.7% among those 6–11, and 22.2% among adolescents 12–19. [1]
Race and ethnicity
Overall, obesity prevalence was highest in Hispanic children (26.2%) and non-Hispanic Black children (24.8%) followed by non-Hispanic white (16.6%) and non-Hispanic Asian (9.0%) children. [1]
Among U.S. girls, obesity prevalence was highest among non-Hispanic Black girls (30.8%). Among U.S. boys, obesity prevalence was highest among Hispanic boys (29.3%). [1]
Family income
Obesity prevalence increased as family income decreased. Obesity prevalence was:
- 11.5% among U.S. children with family income more than 350% of the Federal Poverty Level (FPL).
- 21.2% among children with family income 130% to 350% of FPL.
- 25.8% among children with family income 130% or less of FPL. [1]
Obesity data among young children
Health care for obesity is expensive.
Health care for obesity is expensive for patients and the health care system. In 2019 dollars, the estimated annual medical cost of obesity among U.S. children was $1.3 billion. Medical costs for children with obesity were $116 higher per person per year than for children with healthy weight. Medical costs for children with severe obesity were $310 higher per person per year than for children with healthy weight. [2]
Related information
Adult Obesity Facts
Information about obesity among adults in the U.S.
About Child and Teen BMI
What BMI is, how it is used, and how it is interpreted.
Child and Teen BMI Calculator
Calculate BMI, BMI percentile, and BMI category for children and adolescents 2–19.
Person-first language
- Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes . Natl Health Stat Report . 2021;158.
- Ward ZJ, Bleich S, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One . 2021;16(3):e0247307.
CDC's obesity prevention efforts focus on policy and environmental strategies to make healthy eating and active living accessible for everyone.
For Everyone
Health care providers, public health.
The overall mission of the Duke Center for Childhood Obesity Research (DCCOR) is to advance effective and equitable obesity prevention and treatment by conducting innovative interdisciplinary research to achieve optimal health for all children.
DCCOR conducts groundbreaking research that seeks to change practice and policy to help children lead healthier lives. Three pillars form the foundation for our research:
- Causes, consequences, and correlates of childhood obesity
- Prevention of obesity and its related morbidities
- Treatment of childhood obesity across the lifespan
Across all pillars, we approach research relative to the following intersecting themes, in order to achieve optimal obesity-related health outcomes:
- Reducing stigma and bias
- Improving health equity
- Establishing policy
- Training the next generation of researchers
In support of our overarching mission, the center’s goals are to:
- Embrace innovative research strategies and support interdisciplinary collaboration by intentionally seeking out collaborators across different departments.
- Discover and deliver effective obesity prevention and treatment to populations of children across the age spectrum from pre-conception through early adulthood by identifying the physical, mental, social, and economic factors that affect parents and/or children in ways that lead to weight gain.
- Close disparities in optimal nutrition and activity that exist for children from diverse racial, ethnic, and economic diverse backgrounds by developing culturally-sensitive intervention materials and focus on reducing barriers and facilitating access to opportunities for healthy eating and activity.
- Combat stigma and bias against those affected by obesity by exploring implicit attitudes and their effects on beliefs, behaviors, and health.
- Educate learners at all levels about obesity --its causes and effects, weight stigma, and healthy lifestyles by developing a curriculum for children and/or parents that can be used in schools or other settings.
- Train future leaders in the field of child obesity research by offering training opportunities and mentorship of junior researchers.
- Engage with schools and local health partners to ensure research efforts are community-based by involving non-university and non-academic collaborators at various steps of the research process.
- Encourage the development of policies that will benefit child health and promote healthy lifestyle habits by collaborating with legislators and key decision-makers and providing them with expertise and advice.
- Publicize our research and disseminate the findings to a wide audience through rigorous scientific channels, center-created newsletters and informational resources, and social media platforms.
With the creation of the Duke Center for Childhood Obesity Research (DCCOR) in January 2017 under the leadership of Eliana Perrin, MD, MPH, the Department of Pediatrics and School of Medicine strengthened its commitment to the multidisciplinary research necessary to develop effective and efficient evidence-based behavioral interventions to prevent childhood obesity. Additional Center revenue and project funding is generated by grant awards from sources such as The Duke Endowment and the National Institutes of Health (NIH). The Center is currently co-directed by Sarah Armstrong, MD , director of the Duke Healthy Lifestyles clinical and research programs and Division Chief of General Pediatrics and Adolescent Health in the Department of Pediatrics, and Asheley Skinner, PhD , Professor of Population Health Sciences and Director of Graduate Studies for Population Health Sciences. The Center is conducting impactful, multidisciplinary research on the causes, consequences, correlates, prevention and treatment of childhood obesity.
Research Environment
DCCOR, which is an integral part of the Department of Pediatrics in the Duke University School of Medicine, is strategically positioned to conduct innovative and groundbreaking research in pediatric obesity. Duke University has a strong reputation as one of the top research institutions in the country. In 2021, the Duke School of Medicine received more than $608 million in NIH funding, ranking third in the nation. Ranking first nationally in NIH research grant funding for pediatrics clinical science departments, Duke’s Department of Pediatrics received nearly $210 million in NIH grants in 2021.
Duke University is highly supportive of research collaboration among faculty members across disciplines, departments, and schools. With the introduction of the 2006 strategic plan “Making a Difference,” Duke began to build university-wide interdisciplinary institutes, initiatives, and centers with the intention of taking novel approaches to problem-focused research. These interdisciplinary entities are supported with core funding from the Office of the Provost, sharing infrastructures that facilitate the work being accomplished by the faculty and students within them. Further evidence of Duke’s support of facilitating interdisciplinary partnerships is the School of Medicine’s interdisciplinary colloquia awards, which aim to connect faculty members from a variety departments to share knowledge and collaborate on common interests.
Duke’s expansive research infrastructure and history of supporting interdisciplinary collaborations will provide DCCOR with a strong foundation to successfully engage in innovative research, make critical advancements and discoveries, and become a leader in the field of pediatric obesity research and prevention.
- CLASSIFIEDS
- Advanced search

American Board of Family Medicine
Advanced Search
Socioeconomic Status and Other Factors Associated with Childhood Obesity
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Data
- Info & Metrics
Background: Childhood obesity in the United States is a critical public health issue. Although multiple child and parental factors are associated with childhood obesity, few models evaluate how socioeconomic status influences these risk factors. We aimed to create a model to examine how socioeconomic status modifies risk factors for child obesity.
Methods: We conducted a secondary data analysis of the Early Childhood Longitudinal Birth Cohort. Using logistic regression, we modeled childhood obesity status from known parental and child risk factors for childhood obesity and tested interactions with socioeconomic status.
Results: Compared with healthy-weight children, socioeconomic status, race, birth weight, parental smoking, and not eating dinner as a family were associated with kindergarten-aged children being overweight or obese. Parental smoking increased the odds of a child being overweight or obese by 40%, and eating dinner as a family reduced the odds of a child being overweight or obese by 4%. In addition, black or Hispanic children had a 60% increased odds of being overweight or obese when compared with their white counterparts. Native American children had almost double the odds of being overweight or obese compared with white children. Socioeconomic status did not modify any of these associations.
Conclusion: Parental smoking, birth weight, and not eating dinner as a family were two modifiable factors associated with overweight and obesity in kindergarten-age children, regardless of socioeconomic status. Changing these life-style factors could reduce the child's risk for obesity.
- Pediatric Obesity
- Primary Health Care
- Public Health
- Risk Factors
- Socioeconomic Status
Childhood obesity is a critical public health issue, with one-third of all children and adolescents in the United States being either overweight or obese. 1 Children who are overweight in kindergarten are 4 times more likely than healthy-weight children to be obese at age fourteen. 2 Despite advances in obesity research, insufficient evidence exists about how children develop obesogenic behaviors such as inactivity and poor nutritional preferences, especially in families of low socioeconomic status (SES). Recent studies suggest that children begin to develop health behaviors and attitudes as young as 5 years of age. 3 Children as young as 4 or 5 years old may begin internalizing their parents' physical activity and dietary habits. A previous study shows that 71% of childhood obesity is explained by the influence of family factors on young children. 4 This suggests that early childhood may provide the best window of opportunity for modifying environmental risk factors for childhood obesity.
The etiology of childhood obesity seems to be multifactorial. Child behavioral risk factors known to increase obesity risk include decreased physical activity rates 5 , increased time playing video games and watching television 6 , and being put to bed with a bottle. 7 Some studies also show a strong association between birth weight and childhood obesity. 8 Multiple studies document parental risk factors for their children's overweight status, including maternal obesity 8 , 9 , lower educational attainment 10 , African American race 11 , lower physical activity rates 12 , poor nutrition knowledge 12 , food insecurity 13 , smoking 8 , rules about food consumption and eating at regular times 14 , and perceived neighborhood safety. 14 The increased childhood obesity associated with these risk factors results in poor health outcomes in these children that persist as they become adults through the development of chronic diseases such as diabetes, hypertension, and coronary heart disease. 15
Low family SES is associated with increased childhood obesity rates. Despite recent modest improvements in obesity rates among US low-income, preschool-aged children 16 , obesity rates continue to be higher among low-income children. 17 However, this trend is not consistent in all races and at all SES levels. 18 Some attribute the increased rate of obesity in minorities to their greater poverty rates. 19 However, studies show that black children with higher SES do not exhibit the trend of lower obesity prevalence as do higher SES white children. 18 , 20 , 21 In this study, we used a large national database to evaluate whether SES modifies risk factors for childhood obesity, including race. With this knowledge at hand, health care providers who care for children who are racial minorities can better address their pediatric patients' obesity by understanding the relationship between race, modifiable risk factors, SES, and childhood obesity.
Many modifiable risk factors for childhood obesity are related to SES, including neighborhood safety, smoking, drinking soda, and watching television. 8 Demographic risk factors associated with increased childhood weight, such as race and birth weight, also vary with SES. Indeed, some have assumed that these risk factors are SES indicators rather than risk factors for childhood overweight status that are independent of SES. 22 SES is importantly associated with health, including childhood obesity, but it is difficult to alter. Preventive health researchers and providers would benefit from a model that evaluates whether SES modifies the influence of these risk factors on early childhood weight. With such a model, they could design population-level and individual-level interventions that target modifiable risk factors for childhood obesity. Therefore, this study sought to test SES as a modifying risk factor for other demographic and behavioral obesity risk factors associated with childhood obesity.
The Early Childhood Longitudinal Birth Cohort (ECLS-B) is a nationally representative sample of 14,000 children born in the United States in 2001. The subjects, from diverse socioeconomic and racial backgrounds, were followed during the formative years of birth through kindergarten entry. Parents were surveyed at their child's birth, at 9 months, 24 months, and 4 to 5 years of age. Surveys focused on child health, development, care, and education. In this study, we analyzed data from kindergarten entry (ages 4 to 5 years), which included 7,022 children. The ECLS-B employed a multistage, stratified, and clustered design for data collection. 23 Data were collected at every round by trained assessors who visited the child and parents in their homes. At the visits, assessors measured height and weight, which were used to calculate body mass index (BMI). Children's BMI is classified by standard percentiles; those with a BMI greater than 85% were classified as overweight or obese, according to Centers for Disease Control and Prevention recommendations. 24 The database also includes birth weight from US birth certificate data.
The ECLS-B oversamples children who are twins, Chinese and other Asian and Pacific Islanders, American Indians and Alaska Natives, and those born with low or very low birth weight. 23 Researchers used weighted analysis to offset oversampling. The University of Missouri Health Sciences Institutional Review Board approved this project.
Our study's major outcome measure is the child's weight status (overweight or obese, ≥85th percentile vs healthy weight, <85th percentile, based on age and gender), as measured during the ECLS-B kindergarten-entry visit. Assessors determined food security based on the survey at the same visit. The survey options were food secure, food insecure without hunger, and food insecure with hunger. Assessors also surveyed parents regarding parental household status at kindergarten entry, and we consolidated the categories into (1) 2-parent household or (2) other household status, including single-parent household. Assessors recorded the child's sex at birth as well as birth weight (in grams). We evaluated 6 categories of race ascertained at birth: white, black, Hispanic, Asian, Native American, and other. At 9 months, assessors asked parents if the child was routinely put to bed with a bottle (yes or no). Assessors determined the remainder of the behavioral variables at kindergarten entry. The parent survey included whether they had rules about which foods the child could eat (yes or no), if they had rules about what the child could watch on television (yes or no), how many hours of television the child watched each weekday (continuous variable up to 24 hours), how much soda their child consumed in the past 7 days (0/wk, 1–3/wk, 4–6/wk, 1/d, 2/d, 3/d, 4/d), how many days per week the family eats at a regular time (0 to 7), how safe the parent felt the neighborhood was (very safe, fairly safe, fairly unsafe, very unsafe), and how many days in the past week the parent had exercised for 30 minutes or more (0 to 7). We considered but ultimately excluded the following variables from the model due to too much missing data: participation in free or reduced lunch at school, breastfeeding versus bottle feeding, child's habit of snacking after school, and amount of the child's aerobic exercise.
Household SES is a measure of a family's relative social position. The SES measure is based on 5 equally weighted, standardized components: family income, father's/guardian's educational attainment, mother's/guardian's educational attainment, father's/guardian's prestige of occupation, and mother's/guardian's prestige of occupation. This is a composite variable included in the ECLS database, with the fifth quintile being the highest. Parental education was coded with 9 categories (grades 0 to 11, grade 12 without diploma or equivalent, high school diploma or equivalent, vocational/technical after high school, some college, Bachelor's degree, graduate or professional school but no degree, Master's degree, doctorate or professional degree after bachelor's degree) with the highest level of education in the household recorded and included birth parents, adoptive parents, step parents, and foster parents. Occupation was classified according to the Standard Occupation Classification Manual 25 and collapsed into 23 codes and the alternative codes of retired/unemployed and “uncodable.” Each parent's occupation was scored using the average of the 1989 General Social Survey prestige scores for the 1980 census occupational category codes that correspond to the Early Childhood Longitudinal Study, Kindergarten Class (ECLS-K) occupation code. These prestige scores ranged from 77.5 for physicians/dentists/veterinarians to 29.6 for handlers/equipment cleaners/helpers/laborers. Household income was not relative to household size but rather was comprised of 18 categories that increased by intervals of $5,000 starting at less than $5,000 and increasing to greater than $200,000. Rather than use income markers such as poverty level, we choose SES because it includes parental education and occupation in addition to household income. Thus, it is a more robust variable that may more fully describe then children's home environment. Other researchers have employed the SES composite variable to evaluate children's weight. 26
Statistical Analysis
Simple logistic regression was used to assess the association between overweight status and each individual risk factor. Risk factors with P < .2 in the simple analyses were candidates for inclusion in a multivariable model. Logistic regression with stepwise variable selection was used to identify our main-effects risk model. A significance level of .05 was used as the variable entry and retention criteria in the stepwise process. Odds ratios (ORs) and 95% CIs were calculated. The final phase of the regression analysis was to include 2-way interaction terms involving the selected main effects and SES. Only significant variables were included in the main effects model to test SES interaction term. Statistically significant interactions would suggest that the actual effects of a risk factor are influenced by SES. SAS 9.4 (SAS institute Inc., Cary, NC) was used for all analyses. SAS SURVEY procedures were used to accommodate the complex survey sample design. We conducted the analysis in 2015 and reviewed it in 2016 and 2017.
Of the 7022 children in our analysis, 49.06% were female. The majority of children were white (53.80%), followed by Hispanic (25.12%), black (13.88%), other races (3.94%), Asian (2.62%), and Native American (0.65%). Most of the children (68.96%) resided in 2-parent households, with both their biological mother and father. Overall, 36.31% of children in the sample were overweight or obese ( Table 1 ).
- View inline
Univariable Analyses of Factors Associated with Child Obesity in the Early Childhood Longitudinal Birth Cohort Data
In univariable logistic models, all the variables we tested were significantly and independently associated with children being overweight or obese at kindergarten, except child's sex and number of hours worked by the mother ( Table 1 ). In the multivariable logistic regression analysis, SES, race, birth weight, smoking status, and not eating dinner as a family remained statistically significantly associated with children being overweight or obese ( Table 2 ). Interactions between these variables and SES were not statistically significant, indicating that that SES did not modify the relationship between these variables and childhood obesity ( Table 2 ).
Multiple Logistic Regression Analysis of a Child Being Obese or Overweight at Kindergarten Entry
We found a significant association of SES for children who were overweight or obese, compared with healthy-weight children. Children in the lowest quintile of SES were 70% more likely to be overweight or obese than children in the highest quintile (OR 1.7; 95% CI, 1.3–2.2) Children who were black, Hispanic, Native American, or other races had a statistically significantly increased risk of being overweight or obese compared with white children. Only Asian children had a nonsignificant odds of being overweight or obese compared with white children. Black or Hispanic children had a 60% increased odds of being overweight or obese when compared with their white counterparts. Native American children had an almost doubled odds of being overweight or obese compared with white children ( Table 2 ). Elevated birth weight was significantly associated with overweight status in children (OR 1.07; 95% CI, 1.06–1.08). Every 100-g increase in birth weight was associated with a 7% increased risk of overweight or obesity ( Table 2 ). Parental smoking was associated with 40% higher odds of child overweight or obesity, while eating dinner as a family was associated with 4% lower odds.
We found that race, birth weight, parental smoking, and not having family meals were associated with obesity, but there were no statistically significant interaction terms between SES and any of these variables. This suggests the relationship between these variables and childhood obesity were not modified by SES. This is important because the significant risk factors that are modifiable, birth weight, parental smoking, and not eating meals as a family, could be areas for health care providers to focus anticipatory guidance. Changing the home environment with these modifiable risk factors could indirectly reduce the child's risk for obesity, although it is important to clarify that our study design supports association, not causation.
Our results confirm previous findings that children's weight status in kindergarten varies with SES. 11 The largest odds ratio of being overweight was between the first and the fifth quintiles for SES. Although many believe that food security contributes to increased obesity rates in low-SES families 27 , we did not find that self-reported food security was a significant predictor. Other studies of food security in children similarly show an inconsistent correlation with weight status. 28 ⇓ – 30 This may result from the difficulty of defining and measuring food security. Other factors felt to contribute to obesity in low-SES families include different feeding behaviors 31 , 32 and disproportionately more psychosocial stressors. 33
By using the SES composite variable, which includes household income as well as parents' education and occupation, our study demonstrated that a combination of socioeconomic factors contribute to children's early obesogenic environment. Thus, a more comprehensive approach to childhood obesity could include interventions targeting parents not only of different income levels, but also of different education levels and occupations. 10 We found higher rates of overweight and obesity in black, Native American, and other children in the fourth and fifth quintiles, compared with white children. Because SES did not significantly modify the relationship between race and childhood obesity, other differences should also be considered when addressing childhood obesity. This study supports an increasing body of evidence that racial differences in childhood obesity are not modified by SES. 18 The higher prevalence of childhood obesity among racial minorities is likely the result of complex interactions between a multitude of factors.
Racial neighborhood differences are believed to be one contributing factor. Specifically, living in neighborhoods with higher poverty levels, lower educational levels, and a high proportion of black residents is associated with increased risk of childhood obesity. 34 Although our univariate analysis found that neighborhood safety was a risk factor for child overweight and obesity, it was not significantly associated with child weight in the multivariable logistic regression model. Other racial neighborhood differences beyond perceived safety, such as inclusivity of the residential community and availability of outside play equipment and recreation resources, may also contribute.
Limitations
This study was limited by its use of previously collected data. Although our model included many factors that contribute to childhood obesity, objective data about the child's activity level, calories consumed, or maternal weight were not available. However, our model was consistent with many prior studies on childhood obesity. In addition, we only examined weight at kindergarten entry. Further longitudinal analysis may help determine whether the associations of these factors with increased child weight persist into older childhood.
A particular strength of this study is its large, nationally representative population, which reduces the risk of sampling bias. The large sample also allowed us to detect smaller differences in child overweight due to increased power, increasing the model's sensitivity.
In this ECLS-B secondary data analysis study, we created a regression model from known parental and child risk factors for childhood obesity. SES, race, birth weight, parental smoking, and not eating dinner as a family were associated with overweight and obesity in kindergarten-aged children. SES did not modify the relationship between these variables and childhood obesity. Child health care providers should consider discussing these behaviors with families when addressing childhood obesity. Public health programs that influence risk factors, such as promoting healthy birth weight, reducing parental smoking, and eating meals together as a family, may help improve family health behaviors and environment and thus reduce the risk of childhood obesity.
This article was externally peer reviewed.
Funding: This project was internally funded by the Family and Community Medicine Department at the University of Missouri.
Conflict of interest: none declared.
To see this article online, please go to: http://jabfm.org/content/31/4/514.full .
- Received for publication July 3, 2017.
- Revision received December 8, 2017.
- Accepted for publication December 31, 2017.
- Carroll MD ,
- Cunningham SA ,
- Kramer MR ,
- Flexeder C ,
- Fuertes E ,
- Boonpleng W ,
- McCreary L ,
- Dennison BA ,
- Janjua NZ ,
- Mahmood B ,
- Goldenberg RL
- Melgar-Quinonez HR ,
- Cooksey R ,
- Gravenor MB ,
- Natale RA ,
- Messiah SE ,
- Uhlhorn SB ,
- Delamater A ,
- Toschke AM ,
- Ruckinger S ,
- Blanck HM ,
- Dalenius K ,
- Grummer-Strawn LM
- 17. ↵ Childhood obesity facts: childhood obesity among preschoolers is more prevalent among those from lower-income families . Available from: http://www.cdc.gov/obesity/data/childhood.html#obesity-among-preschoolers . Published 2014 . Accessed 7 March 2016 .
- Obarzanek E ,
- Barton BA ,
- Okosun IS ,
- Fradkin C ,
- Wallander JL ,
- Elliott MN ,
- Tortolero S ,
- Cuccaro P ,
- Schuster MA
- 24. ↵ Basics about childhood obesity . 2012 . Available from: http://www.cdc.gov/obesity/childhood/basics.html . Published 2012 . Accessed 10 November 2014 .
- 25. ↵ Office of the President, Office of Management and Budget . Standard occupational classification manual . U.S. Department of Commerce, Technology Administration, National Technical Information Service : Springfield, VA ; 2000 .
- Jones-Smith JC ,
- Dieckmann MG ,
- Gottlieb L ,
- Bhargava A ,
- Jolliffe D ,
- Whitaker RC ,
- Gundersen C ,
- Garasky S ,
- Murashima M ,
- Hughes SO ,
- Kaplowitz SA
- Papaioannou MA ,
- Mahatmya D ,
- Kimbro RT ,
In this issue

- Table of Contents
- Table of Contents (PDF)
- Cover (PDF)
- Index by author
Thank you for your interest in spreading the word on American Board of Family Medicine.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Related articles.
- No related articles found.
- Google Scholar

Cited By...
- Associations of longitudinal BMI percentile classification patterns in early childhood with neighborhood-level social determinants of health
- Food Intake Compared to Exercise Association with Obesity in Children Ages 3-6
- The Most Frequently Read Articles of 2020
- Content Usage and the Most Frequently Read Articles of 2018
- The Potpourri of Family Medicine, in Sickness and in Health
More in this TOC Section
- Barriers and Facilitators to Using a Clinical Decision Support Tool for Opioid Use Disorder in Primary Care
- How An Academic Direct Primary Care Clinic Served Patients from Vulnerable Communities
- Relationship Between Primary Care Physician Capacity and Usual Source of Care
Similar Articles
Search form
Evaluating the benefits of and barriers to pediatric obesity programs.

(© stock.adobe.com)
Obesity now affects more than one in five children in the United States, and while there are effective, recommended interventions, availability is limited for most children. In two new studies, Yale researchers assessed the cost-effectiveness of one intervention and factors that have hindered and facilitated implementation of another to uncover strategies for improving access to effective pediatric obesity treatment.
The publications are timely as Yale experts, working as members of national medical organizations, have supported a proposal under consideration by the Centers for Medicare and Medicaid Services for a new billing code that could allow facilities to be reimbursed by health insurance for intensive health behavior and lifestyle treatment interventions for childhood obesity. Such a change would thereby encourage implementation of these programs and improve access to them, the researchers say.
The studies were published Aug. 28 in the journal Obesity.
Previous research has shown that interventions that provide comprehensive, family-centered nutrition and behavioral education, and at least 26 contact hours with families over 3 to 12 months, are effective at treating childhood obesity. These types of programs have been recommended by both the U.S. Preventative Service Task Force and the American Academy of Pediatrics.
“ We have treatment options that work,” said Mona Sharifi , an author of both studies and an associate professor of pediatrics at Yale School of Medicine. “But we have these systematic barriers to access that we need to address rapidly.”
Cost is a perennial concern affecting health care programs, obesity treatments included. In the first new study, Sharifi and her colleagues evaluated the costs — from both a health care and a societal perspective — associated with implementing the Healthy Weight Clinic intervention in federally qualified health centers.
The Healthy Weight Clinic is a program that delivers intensive health behavior and lifestyle treatment for children and adolescents with obesity or overweight that is consistent with guidelines from the American Academy of Pediatrics. The treatment model brings together teams of pediatricians, dieticians, and community health workers within primary care settings where families are already likely to be engaged.
For the first new study , the researchers looked at federally qualified health centers specifically, as they provide services in underserved communities.
“ This was purposeful to access communities that are disproportionately affected by obesity disparities,” said Sharifi.
In their analysis, the researchers broke down the intervention to its smallest pieces — personnel, materials, etc. — and determined their costs. They also estimated costs incurred by families in the form of time, transportation, and childcare expenses associated with participating in a Healthy Weight Clinic. They then entered those costs into a model that simulated a sample of patients over a 10-year period, some of whom entered a Healthy Weight Clinic intervention.
“ We were able to extrapolate those calculations out and ask, if we were able to spread this intervention to all eligible federally qualified health centers in the U.S., what would the scene look like in 10 years?” said Sharifi. “How many cases of obesity would we prevent? How much would it cost and how much might we save by improving the health of children reached by the intervention?”
They found that if Healthy Weight Clinics were made available in all federally qualified health centers over 10 years, the intervention would reach 888,000 children with obesity or overweight and prevent 12,100 cases of obesity and 7,080 cases of severe obesity.
Costs were estimated at $667 per child reached — with $456 paid by the health care sector and $211 incurred by families. Over the same time, however, the reduction in obesity cases would save approximately $14.6 million dollars in health care costs.
“ It’s a relatively low-cost intervention that our study team previously found to be effective,” said Sharifi. “And given the populations federally qualified health centers serve, our findings also project that scaling up this intervention could mitigate health inequities affecting underserved populations.”
In the second study , the researchers evaluated another intervention, by studying the dissemination of a curriculum called Smart Moves that came out of a Yale-developed program named Bright Bodies. Previous research from Sharifi, Mary Savoye (the founder of Smart Moves), and their colleagues has shown Bright Bodies to be both effective at improving health outcomes in children with obesity and overweight and, compared with usual clinical care, cost-saving .
From 2003 to 2018, the SmartMoves curriculum was disseminated to over 30 U.S.-based sites. The new study collected experiences from staff that worked at those sites to identify what factors facilitated the program’s implementation and what barriers exist to its success.
Two of the strongest facilitators of SmartMoves implementation were local partnerships with schools and exercise facilities that helped provide resources and demand for programming from families.
The biggest barrier to sustainability was funding insecurity; more often than not, this barrier resulted in failed efforts to implement or sustain new programs.
“ When a child breaks their arm, the family seeks care, and the clinic or hospital bills their insurance company to cover the cost of treatment. This model of funding doesn’t work as well for health behavior and lifestyle treatment programs,” said Sharifi. “For example, Bright Bodies involves group visits with families and is run by a dietician, an exercise physiologist, and a social worker. So you typically can’t get reimbursement from insurance companies even though Bright Bodies appears to be more effective and cost saving compared with usual clinical care. These programs often rely on grants, but grants run out and programs disappear, leaving communities lacking access to standard of care treatment.”
To pave the way for effective programs like Bright Bodies and Healthy Weight Clinic to receive reimbursement, several organizations including the American Academy of Pediatrics, the American Academy of Family Physicians, and the U.S. Centers for Disease Control and Prevention, submitted an application that would establish a new billing code. The proposal will be deliberated over the next few months by the Centers for Medicare and Medicaid Services.
“ If approved, I think it would open the door to funding the most efficient and appropriate way to deliver this treatment and give families more options for interventions,” said Sharifi. “This kind of thing — treatment that is standard of care not being reimbursed — would never happen in a field like surgery. But it happens in pediatrics because children often get neglected in U.S. health care policy and pediatricians often get shortchanged in billing.”
Policy change, she said, is needed to ensure this first-line treatment is accessible to families throughout the country.
“ Expanding access is an urgent need,” said Sharifi. “And not providing equitable access to effective, low-cost treatment for children is unethical.”
Health & Medicine
Media Contact
Fred Mamoun: [email protected] , 203-436-2643

Seeing peer ratings pushes professionals to align their evaluations

Yale Planetary Solutions seed grants nurture innovative projects campuswide

An ancient Judaism scholar uncovering forgotten aspects of Jerusalem’s past

Yale Film Archive offers 21 screenings through December
- Show More Articles
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /
Noncommunicable diseases: Childhood overweight and obesity
The prevalence of overweight and obesity in adolescents is defined according to the WHO growth reference for school-aged children and adolescents (overweight = 1 standard deviation body mass index for age and sex, and obese = 2 standard deviations body mass index for age and sex).
Overweight and obesity are defined as ''abnormal or excessive fat accumulation that presents a risk to health''.
Childhood obesity is one of the most serious public health challenges of the 21st century. The problem is global and is steadily affecting many low- and middle-income countries, particularly in urban settings. The prevalence has increased at an alarming rate. Globally in 2016, the number of overweight children under the age of five, is estimated to have been over 41 million. Almost half of all overweight children under 5 lived in Asia and one quarter lived in Africa. Overweight and obese children are likely to stay obese into adulthood and more likely to develop noncommunicable diseases like diabetes and cardiovascular diseases at a younger age. Overweight and obesity, as well as their related diseases, are largely preventable. Prevention of childhood obesity therefore needs high priority.
The most commonly used measure for overweight and obesity is the Body Mass Index (BMI) - a simple index to classify overweight and obesity in adults. It is defined as the weight in kilograms divided by the square of the height in meters (kg/m2).
The BMI provides the most useful population-level measure of overweight and obesity, as it is the same for both sexes and for all ages of adults. However, it should be considered as a rough guide because it may not correspond to the same body fat percentage in different individuals.
It is difficult to develop one simple index for the measurement of overweight and obesity in children and adolescents because their bodies undergo a number of physiological changes as they grow. Depending on the age, different methods to measure a body's healthy weight are available:
For children aged 0-5 years
The WHO Child Growth Standards, launched in April 2006, include measures for overweight and obesity for infants and young children up to age 5.
- The WHO Child Growth Standards
- WHO Global Database on Child Growth and Malnutrition, 0-5 years.
For individuals aged 5-19 years
WHO developed the Growth Reference Data for 5–19 years. It is a reconstruction of the 1977 National Center for Health Statistics (NCHS)/WHO reference and uses the original NCHS data set supplemented with data from the WHO child growth standards sample for young children up to age 5.
- Growth reference data for 5-19 years
- Global school-based student health survey (GSHS)
Childhood obesity is associated with a higher chance of premature death and disability in adulthood. Overweight and obese children are more likely to stay obese into adulthood and to develop noncommunicable diseases (NCDs) like diabetes and cardiovascular diseases at a younger age. For most NCDs resulting from obesity, the risks depend partly on the age of onset and on the duration of obesity. Obese children and adolescents suffer from both short-term and long-term health consequences.
The most significant health consequences of childhood overweight and obesity, which often do not become apparent until adulthood, include:
- cardiovascular diseases (mainly heart disease and stroke);
- musculoskeletal disorders, especially osteoarthritis; and
- certain types of cancer (endometrial, breast and colon).
At least 2.6 million people each year die as a result of being overweight or obese.
Many low- and middle-income countries are now facing the so-called double burden of disease. As they continue to struggle with the problems of infectious diseases and under-nutrition, at the same time they are experiencing a rapid increase in risk factors of NCDs such as obesity and overweight, particularly in urban settings.
It is not uncommon to find under-nutrition and obesity existing side by side within the same country, the same community or even within the same household in these settings.
This double burden is caused by inadequate prenatal, infant and child nutrition, which is then followed by exposure to high fat, energy dense, micronutrient poor foods and a lack of physical activity as the child grows older.
The fundamental cause of childhood overweight and obesity is an energy imbalance between calories consumed and calories expended.
Global increases in childhood overweight and obesity are attributable to several factors. First, there has been a global shift in diet towards increased intake of energy-dense foods that are high in fat and sugars but low in vitamins, minerals and other healthy micronutrients. There is also a trend towards decreased physical activity levels due to the increasingly sedentary nature of many forms of recreation time, changing modes of transportation and increasing urbanization.
WHO recognizes that the increasing prevalence of childhood obesity results from changes in society. Childhood obesity is mainly associated with unhealthy eating and low levels of physical activity, but the problem is linked not only to children's behaviour but also, increasingly, to social and economic development and policies in the areas of agriculture, transport, urban planning, the environment, food processing, distribution and marketing, as well as education.
The problem is societal and therefore it demands a population-based multisectoral, multidisciplinary and culturally relevant approach.
Unlike most adults, children and adolescents cannot choose the environment in which they live or the food they eat. They also have a limited ability to understand the long-term consequences of their behaviour. They therefore require special attention when fighting the obesity epidemic.
Overweight and obesity, as well as related noncommunicable diseases, are largely preventable. It is recognized that prevention is the most feasible option for curbing the childhood obesity epidemic since current treatment practices are largely aimed at bringing the problem under control rather than effecting a cure. The goal in fighting the childhood obesity epidemic is to achieve an energy balance which can be maintained throughout the individual's life span.
WHO recommends the following to reduce and prevent childhood overweight and obesity:
- increase consumption of fruit and vegetables, as well as legumes, whole grains and nuts;
- limit energy intake from total fats and shift fat consumption away from saturated fats to unsaturated fats;
- limit the intake of sugars; and
- be physically active and accumulate at least 60 minutes of regular, moderate- to vigorous-intensity activity each day that is developmentally appropriate.
Curbing the childhood obesity epidemic requires sustained political commitment and the collaboration of many public and private stakeholders.
Governments, international partners, civil society, NGOs and the private sector have vital roles to play in shaping healthy environments and making healthier diet options for children and adolescents affordable, and easily accessible. It is therefore WHO's objective to mobilize these partners and engage them in implementing the Global Strategy on Diet, Physical Activity and Health.
WHO supports the designation, implementation, monitoring and leadership of actions. A multisectoral approach is essential for sustained progress; the Organization mobilizes the combined energy, resources and expertise of all global stakeholders involved.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 28, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Evaluating the benefits of and barriers to pediatric obesity programs
by Mallory Locklear, Yale University
Obesity now affects more than one in five children in the United States, and while there are effective, recommended interventions, availability is limited for most children. In two new studies, Yale researchers assessed the cost-effectiveness of one intervention and factors that have hindered and facilitated implementation of another to uncover strategies for improving access to effective pediatric obesity treatment.
The publications are timely as Yale experts, working as members of national medical organizations, have supported a proposal under consideration by the Centers for Medicare and Medicaid Services for a new billing code that could allow facilities to be reimbursed by health insurance for intensive health behavior and lifestyle treatment interventions for childhood obesity . Such a change would thereby encourage implementation of these programs and improve access to them, the researchers say.
The studies were published in the journal Obesity .
Previous research has shown that interventions that provide comprehensive, family-centered nutrition and behavioral education, and at least 26 contact hours with families over 3 to 12 months, are effective at treating childhood obesity. These types of programs have been recommended by both the U.S. Preventative Service Task Force and the American Academy of Pediatrics.
"We have treatment options that work," said Mona Sharifi, an author of both studies and an associate professor of pediatrics at Yale School of Medicine. "But we have these systematic barriers to access that we need to address rapidly."
Cost is a perennial concern affecting health care programs, obesity treatments included. In the first new study, Sharifi and her colleagues evaluated the costs—from both a health care and a societal perspective—associated with implementing the Healthy Weight Clinic intervention in federally qualified health centers.
The Healthy Weight Clinic is a program that delivers intensive health behavior and lifestyle treatment for children and adolescents with obesity or overweight that is consistent with guidelines from the American Academy of Pediatrics. The treatment model brings together teams of pediatricians, dieticians, and community health workers within primary care settings where families are already likely to be engaged.
For the first new study , the researchers looked at federally qualified health centers specifically, as they provide services in underserved communities.
"This was purposeful to access communities that are disproportionately affected by obesity disparities," said Sharifi.
In their analysis, the researchers broke down the intervention to its smallest pieces—personnel, materials, etc.—and determined their costs. They also estimated costs incurred by families in the form of time, transportation, and childcare expenses associated with participating in a Healthy Weight Clinic. They then entered those costs into a model that simulated a sample of patients over a 10-year period, some of whom entered a Healthy Weight Clinic intervention.
"We were able to extrapolate those calculations out and ask, if we were able to spread this intervention to all eligible federally qualified health centers in the U.S., what would the scene look like in 10 years?" said Sharifi. "How many cases of obesity would we prevent? How much would it cost and how much might we save by improving the health of children reached by the intervention?"
They found that if Healthy Weight Clinics were made available in all federally qualified health centers over 10 years, the intervention would reach 888,000 children with obesity or overweight and prevent 12,100 cases of obesity and 7,080 cases of severe obesity.
Costs were estimated at $667 per child reached—with $456 paid by the health care sector and $211 incurred by families. Over the same time, however, the reduction in obesity cases would save approximately $14.6 million dollars in health care costs .
"It's a relatively low-cost intervention that our study team previously found to be effective," said Sharifi. "And given the populations federally qualified health centers serve, our findings also project that scaling up this intervention could mitigate health inequities affecting underserved populations."
In the second study , the researchers evaluated another intervention , by studying the dissemination of a curriculum called Smart Moves that came out of a Yale-developed program named Bright Bodies. Previous research from Sharifi, Mary Savoye (the founder of Smart Moves), and their colleagues has shown Bright Bodies to be both effective at improving health outcomes in children with obesity and overweight, and compared with usual clinical care, cost-saving .
From 2003 to 2018, the SmartMoves curriculum was disseminated to over 30 U.S.-based sites. The new study collected experiences from staff that worked at those sites to identify what factors facilitated the program's implementation and what barriers exist to its success.
Two of the strongest facilitators of SmartMoves implementation were local partnerships with schools and exercise facilities that helped provide resources and demand for programming from families.
The biggest barrier to sustainability was funding insecurity; more often than not, this barrier resulted in failed efforts to implement or sustain new programs.
"When a child breaks their arm, the family seeks care, and the clinic or hospital bills their insurance company to cover the cost of treatment. This model of funding doesn't work as well for health behavior and lifestyle treatment programs," said Sharifi. "For example, Bright Bodies involves group visits with families and is run by a dietician, an exercise physiologist, and a social worker. So you typically can't get reimbursement from insurance companies even though Bright Bodies appears to be more effective and cost saving compared with usual clinical care. These programs often rely on grants, but grants run out and programs disappear, leaving communities lacking access to standard of care treatment."
To pave the way for effective programs like Bright Bodies and Healthy Weight Clinic to receive reimbursement, several organizations including the American Academy of Pediatrics, the American Academy of Family Physicians, and the U.S. Centers for Disease Control and Prevention, submitted an application that would establish a new billing code. The proposal will be deliberated over the next few months by the Centers for Medicare and Medicaid Services.
"If approved, I think it would open the door to funding the most efficient and appropriate way to deliver this treatment and give families more options for interventions," said Sharifi. "This kind of thing—treatment that is standard of care not being reimbursed—would never happen in a field like surgery. But it happens in pediatrics because children often get neglected in U.S. health care policy and pediatricians often get shortchanged in billing."
Policy change, she said, is needed to ensure this first-line treatment is accessible to families throughout the country.
"Expanding access is an urgent need," said Sharifi. "And not providing equitable access to effective, low-cost treatment for children is unethical."
Emily Benjamin Finn et al, Improving access to first‐line treatment for pediatric obesity: Lessons from the dissemination of SmartMoves, Obesity (2024). DOI: 10.1002/oby.24107
Explore further
Feedback to editors

Brain training: Study links cardiovascular fitness to brain health

Managing early stages of abortion care at home after 12 weeks is safe and reduces time spent in hospital, study finds
12 hours ago

Billions worldwide consume inadequate levels of micronutrients critical to human health, new study finds

Blocking the longevity gene S6K1 extends lifespan in mice by reducing inflammation
13 hours ago

Machine learning predicts which patients will continue taking opioids after hand surgery
14 hours ago

Study describes a new molecular pathway involved in the control of reproduction

Machine learning helps identify rheumatoid arthritis subtypes
15 hours ago

Immune protection against tuberculosis reinfection driven by cells that dampen lung inflammation, shows study

Girls with mental health conditions have lower HPV vaccination coverage

Mankai plant found to reduce post-meal sugar levels in diabetics
16 hours ago
Related Stories

Real‐world effectiveness of a healthy lifestyle intervention for childhood obesity
Feb 23, 2023

American Academy of Pediatrics issues guidelines for children with overweight, obesity
Jan 10, 2023

Childhood weight management program effective for Hispanic children from low-income families
Sep 8, 2021

Preventing childhood obesity requires changes in parents' and clinicians' early-life care
Jul 29, 2021
Program led by health coaches at primary care clinics helped reduce heart risk
Feb 9, 2021

Telehealth study investigates reimbursements for rural health care delivery
Mar 15, 2024
Recommended for you

Preventive vaccination could be a key strategy against Lassa fever and the next pandemic
Aug 28, 2024

Sharing expands caring: Study finds solution to a major source of doctor burnout
Aug 26, 2024

Study finds nearly half of US counties have at least one 'pharmacy desert'

Good sleep habits important for overweight adults, study suggests
Aug 23, 2024

Weight loss drug's heart benefits extend to people with heart failure, study finds
Aug 22, 2024

Clinical trial in Ireland challenges beliefs about Ozempic and similar new obesity treatments
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases & Disorders Education Program
- Publications and Resources
- Clinical Trials
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Ask a Scientist
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Research Topics
Obesity Research
Language switcher.
Over the years, NHLBI-supported research on overweight and obesity has led to the development of evidence-based prevention and treatment guidelines for healthcare providers. NHLBI research has also led to guidance on how to choose a behavioral weight loss program.
Studies show that the skills learned and support offered by these programs can help most people make the necessary lifestyle changes for weight loss and reduce their risk of serious health conditions such as heart disease and diabetes.
Our research has also evaluated new community-based programs for various demographics, addressing the health disparities in overweight and obesity.
NHLBI research that really made a difference
- In 1991, the NHLBI developed an Obesity Education Initiative to educate the public and health professionals about obesity as an independent risk factor for cardiovascular disease and its relationship to other risk factors, such as high blood pressure and high blood cholesterol. The initiative led to the development of clinical guidelines for treating overweight and obesity.
- The NHLBI and other NIH Institutes funded the Obesity-Related Behavioral Intervention Trials (ORBIT) projects , which led to the ORBIT model for developing behavioral treatments to prevent or manage chronic diseases. These studies included families and a variety of demographic groups. A key finding from one study focuses on the importance of targeting psychological factors in obesity treatment.
Current research funded by the NHLBI
The Division of Cardiovascular Sciences , which includes the Clinical Applications and Prevention Branch, funds research to understand how obesity relates to heart disease. The Center for Translation Research and Implementation Science supports the translation and implementation of research, including obesity research, into clinical practice. The Division of Lung Diseases and its National Center on Sleep Disorders Research fund research on the impact of obesity on sleep-disordered breathing.
Find funding opportunities and program contacts for research related to obesity and its complications.
Current research on obesity and health disparities
Health disparities happen when members of a group experience negative impacts on their health because of where they live, their racial or ethnic background, how much money they make, or how much education they received. NHLBI-supported research aims to discover the factors that contribute to health disparities and test ways to eliminate them.
- NHLBI-funded researchers behind the RURAL: Risk Underlying Rural Areas Longitudinal Cohort Study want to discover why people in poor rural communities in the South have shorter, unhealthier lives on average. The study includes 4,000 diverse participants (ages 35–64 years, 50% women, 44% whites, 45% Blacks, 10% Hispanic) from 10 of the poorest rural counties in Kentucky, Alabama, Mississippi, and Louisiana. Their results will support future interventions and disease prevention efforts.
- The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is looking at what factors contribute to the higher-than-expected numbers of Hispanics/Latinos who suffer from metabolic diseases such as obesity and diabetes. The study includes more than 16,000 Hispanic/Latino adults across the nation.
Find more NHLBI-funded studies on obesity and health disparities at NIH RePORTER.

Read how African Americans are learning to transform soul food into healthy, delicious meals to prevent cardiovascular disease: Vegan soul food: Will it help fight heart disease, obesity?
Current research on obesity in pregnancy and childhood
- The NHLBI-supported Fragile Families Cardiovascular Health Follow-Up Study continues a study that began in 2000 with 5,000 American children born in large cities. The cohort was racially and ethnically diverse, with approximately 40% of the children living in poverty. Researchers collected socioeconomic, demographic, neighborhood, genetic, and developmental data from the participants. In this next phase, researchers will continue to collect similar data from the participants, who are now young adults.
- The NHLBI is supporting national adoption of the Bright Bodies program through Dissemination and Implementation of the Bright Bodies Intervention for Childhood Obesity . Bright Bodies is a high-intensity, family-based intervention for childhood obesity. In 2017, a U.S. Preventive Services Task Force found that Bright Bodies lowered children’s body mass index (BMI) more than other interventions did.
- The NHLBI supports the continuation of the nuMoM2b Heart Health Study , which has followed a diverse cohort of 4,475 women during their first pregnancy. The women provided data and specimens for up to 7 years after the birth of their children. Researchers are now conducting a follow-up study on the relationship between problems during pregnancy and future cardiovascular disease. Women who are pregnant and have obesity are at greater risk than other pregnant women for health problems that can affect mother and baby during pregnancy, at birth, and later in life.
Find more NHLBI-funded studies on obesity in pregnancy and childhood at NIH RePORTER.
Learn about the largest public health nonprofit for Black and African American women and girls in the United States: Empowering Women to Get Healthy, One Step at a Time .
Current research on obesity and sleep
- An NHLBI-funded study is looking at whether energy balance and obesity affect sleep in the same way that a lack of good-quality sleep affects obesity. The researchers are recruiting equal numbers of men and women to include sex differences in their study of how obesity affects sleep quality and circadian rhythms.
- NHLBI-funded researchers are studying metabolism and obstructive sleep apnea . Many people with obesity have sleep apnea. The researchers will look at the measurable metabolic changes in participants from a previous study. These participants were randomized to one of three treatments for sleep apnea: weight loss alone, positive airway pressure (PAP) alone, or combined weight loss and PAP. Researchers hope that the results of the study will allow a more personalized approach to diagnosing and treating sleep apnea.
- The NHLBI-funded Lipidomics Biomarkers Link Sleep Restriction to Adiposity Phenotype, Diabetes, and Cardiovascular Risk study explores the relationship between disrupted sleep patterns and diabetes. It uses data from the long-running Multiethnic Cohort Study, which has recruited more than 210,000 participants from five ethnic groups. Researchers are searching for a cellular-level change that can be measured and can predict the onset of diabetes in people who are chronically sleep deprived. Obesity is a common symptom that people with sleep issues have during the onset of diabetes.
Find more NHLBI-funded studies on obesity and sleep at NIH RePORTER.

Learn about a recent study that supports the need for healthy sleep habits from birth: Study finds link between sleep habits and weight gain in newborns .
Obesity research labs at the NHLBI
The Cardiovascular Branch and its Laboratory of Inflammation and Cardiometabolic Diseases conducts studies to understand the links between inflammation, atherosclerosis, and metabolic diseases.
NHLBI’s Division of Intramural Research , including its Laboratory of Obesity and Aging Research , seeks to understand how obesity induces metabolic disorders. The lab studies the “obesity-aging” paradox: how the average American gains more weight as they get older, even when food intake decreases.
Related obesity programs and guidelines
- Aim for a Healthy Weight is a self-guided weight-loss program led by the NHLBI that is based on the psychology of change. It includes tested strategies for eating right and moving more.
- The NHLBI developed the We Can! ® (Ways to Enhance Children’s Activity & Nutrition) program to help support parents in developing healthy habits for their children.
- The Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures Project standardizes data collected from the various studies of obesity treatments so the data can be analyzed together. The bigger the dataset, the more confidence can be placed in the conclusions. The main goal of this project is to understand the individual differences between people who experience the same treatment.
- The NHLBI Director co-chairs the NIH Nutrition Research Task Force, which guided the development of the first NIH-wide strategic plan for nutrition research being conducted over the next 10 years. See the 2020–2030 Strategic Plan for NIH Nutrition Research .
- The NHLBI is an active member of the National Collaborative on Childhood Obesity (NCCOR) , which is a public–private partnership to accelerate progress in reducing childhood obesity.
- The NHLBI has been providing guidance to physicians on the diagnosis, prevention, and treatment of obesity since 1977. In 2017, the NHLBI convened a panel of experts to take on some of the pressing questions facing the obesity research community. See their responses: Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents (PDF, 3.69 MB).
- In 2021, the NHLBI held a Long Non-coding (lnc) RNAs Symposium to discuss research opportunities on lnc RNAs, which appear to play a role in the development of metabolic diseases such as obesity.
- The Muscatine Heart Study began enrolling children in 1970. By 1981, more than 11,000 students from Muscatine, Iowa, had taken surveys twice a year. The study is the longest-running study of cardiovascular risk factors in children in the United States. Today, many of the earliest participants and their children are still involved in the study, which has already shown that early habits affect cardiovascular health later in life.
- The Jackson Heart Study is a unique partnership of the NHLBI, three colleges and universities, and the Jackson, Miss., community. Its mission is to discover what factors contribute to the high prevalence of cardiovascular disease among African Americans. Researchers aim to test new approaches for reducing this health disparity. The study incudes more than 5,000 individuals. Among the study’s findings to date is a gene variant in African Americans that doubles the risk of heart disease.
Explore more NHLBI research on overweight and obesity
The sections above provide you with the highlights of NHLBI-supported research on overweight and obesity . You can explore the full list of NHLBI-funded studies on the NIH RePORTER .
To find more studies:
- Type your search words into the Quick Search box and press enter.
- Check Active Projects if you want current research.
- Select the Agencies arrow, then the NIH arrow, then check NHLBI .
If you want to sort the projects by budget size — from the biggest to the smallest — click on the FY Total Cost by IC column heading.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Parenting and childhood obesity: Validation of a new questionnaire and evaluation of treatment effects during the preschool years
Maria Somaraki
1 Department of Food Studies, Nutrition and Dietetics, Uppsala University, Uppsala, Sweden
2 Division of Pediatrics, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden
3 Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, United Kingdom
Sofia Ljung
4 Aleris Rehab, Skärholmen, Sweden
Veronica Mildton
Pernilla sandvik, paulina nowicka, associated data.
All relevant data are within the paper and its Supporting Information files.
Parenting is an integral component of obesity treatment in early childhood. However, the link between specific parenting practices and treatment effectiveness remains unclear. This paper introduces and validates a new parenting questionnaire and evaluates mothers’ and fathers’ parenting practices in relation to child weight status during a 12-month childhood obesity treatment trial.
First, a merged school/clinical sample (n = 558, 82% mothers) was used for the factorial and construct validation of the new parenting questionnaire. Second, changes in parenting were evaluated using clinical data from the More and Less Study, a randomized controlled trial (RCT) with 174 children (mean age = 5 years, mean Body Mass Index Standard Deviation Score (BMI SDS) = 3.0) comparing a parent support program (with and without booster sessions) and standard treatment. Data were collected at four time points over 12 months. We used linear mixed models and mediation models to investigate associations between changes in parenting practices and treatment effects.
The validation of the questionnaire (9 items; responses on a 5-point Likert scale) revealed two dimensions of parenting (Cronbach’s alpha ≥0.7): setting limits to the child and regulating one’s own emotions when interacting with the child, both of which correlated with feeding practices and parental self-efficacy. We administered the questionnaire to the RCT participants. Fathers in standard treatment increased their emotional regulation compared to fathers in the parenting program (p = 0.03). Mothers increased their limit-setting regardless of treatment allocation (p = 0.01). No treatment effect was found on child weight status through changes in parenting practices.
Taken together, the findings demonstrate that the new questionnaire assessing parenting practices proved valid in a 12-month childhood obesity trial. During treatment, paternal and maternal parenting practices followed different trajectories, though they did not mediate treatment effects on child weight status. Future research should address the pathways whereby maternal and paternal parenting practices affect treatment outcomes, such as child eating behaviors and weight status.
Introduction
Parents are integral to obesity treatment during the preschool years [ 1 , 2 ]. Treatment approaches focus on promoting children’s health behaviors, with the aim of improving children’s energy balance and weight status [ 3 , 4 ]. Thus, healthy eating and exercise are central components of treatment. Nevertheless, these behaviors need to be practiced in a supportive family environment, which is strongly influenced by parenting practices [ 1 , 5 ].
Parenting is commonly conceptualized according to two dimensions, demandingness and responsiveness [ 6 ], which identify four parenting styles (authoritative, authoritarian, neglectful, and indulgent/permissive). These styles account for unique combinations of high and low endorsement of each dimension in relation to the other one [ 7 ]. Demandingness identifies parents’ control and provision of structure, while responsiveness identifies parents’ consideration of a child’s needs. The parenting style described as authoritative, which ranks high in both dimensions (demandingness and responsiveness), has consistently been associated with favorable child behaviors and health outcomes including a healthy weight gain [ 8 , 9 ]. However, most studies have focused on mothers, while the role of fathers remains largely unexplored [ 9 ]. This is a concern in light of fathers’ unique contributions to their children’s health [ 10 , 11 ]. In particular, longitudinal data from Australia suggest that higher paternal responsiveness is associated with increased risk for obesity in early childhood, while similar associations have not been identified for any dimension of maternal parenting [ 12 ]. Therefore, a comprehensive assessment of parenting in relation to childhood obesity should take into account both parents.
While active parental involvement is required for effective obesity treatment in childhood [ 1 , 2 ], little is known about how parents facilitate child weight loss [ 5 , 13 , 14 ]. Effective treatment programs integrate essential education on nutrition and physical activity with guidance for parents on how to maintain children’s healthy behaviors [ 15 – 17 ]. This parent-focused approach is especially relevant during the preschool years, when parents have the greatest capacity to implement changes in the home environment and affect child weight status [ 18 ]. Indeed, obesity treatment among younger children is associated with clinically significant weight loss [ 19 – 21 ]. Reinehr et al. [ 19 ] in Germany evaluated an intensive 1-year long treatment program (without a control condition), which included parenting sessions and aimed to modify lifestyle factors. The study demonstrated that younger children (4–7 years old) maintained their weight loss after 4 years of follow-up to a greater degree than older children and adolescents. To explain these findings, the authors pointed to the fact that parents of younger children were the recipients of the program and bore the main responsibility for the implementation of lifestyle changes. Yet, well-designed randomized controlled trials (RCTs) for childhood obesity in the preschool years are still lacking, and research on programs that address parenting is particularly scarce [ 21 , 22 ].
The present paper attempts to address this gap in the literature by evaluating parenting practices and their effects on child weight status during a 12-month follow-up of an RCT for obesity treatment among preschoolers, the More and Less study (ML study) in Sweden [ 23 ]. The ML study compares a parenting program with and without booster sessions (follow-up phone calls provided after the program, averaging 4 phone calls per family) and standard treatment; the primary study outcome is change in child weight status (body mass index standard deviation score, BMI SDS). The primary findings at 1-year post-baseline showed a greater decrease in child weight status among families randomized to the parenting program with booster sessions, compared to standard care and the parenting program without booster sessions [ 24 ]. In addition, the ML study includes a diverse sample with a high proportion of parents reporting foreign background (parent and/or their parents born outside Sweden) and lower educational attainment, unlike the majority of studies in the field which have included homogeneous samples [ 25 ]. Parental foreign background moderated treatment effects; specifically, boosters were necessary for sustained treatment effects among children whose parent(s) had foreign background [ 24 ]. The parenting program draws on the Oregon Model of Behavior Family Therapy, which emphasizes a core set of evidence-based parenting skills, i.e. encouragement, monitoring, limit-setting strategies, positive involvement, problem solving, and emotional regulation [ 26 – 28 ]. Thus, the parenting program focuses on important aspects of demandingness (e.g. limit setting) and responsiveness (e.g. emotional regulation), which compose the favorable authoritative parenting style with regard to childhood obesity [ 9 , 29 ]. While parenting programs unrelated to obesity have addressed child behaviors in randomized studies successfully [ 30 , 31 ], they have rarely been applied in the field of early childhood obesity [ 21 ]. The relevance of parenting skills in early childhood obesity, however, has been highlighted in observational studies [ 32 , 33 ]. In addition to the parenting practices, the ML program offers developmentally appropriate information around healthy lifestyles (nutrition and physical activity) [ 23 ]. To evaluate the central parenting components of the ML program (encouragement, monitoring, limit-setting strategies, positive involvement, problem solving, and emotional regulation) a valid user-friendly tool was required. Given the lack of appropriate evaluation instruments, we developed a questionnaire to assess changes in parental behaviors after participating in the ML program.
This paper has a two-fold aim. First, to validate a new questionnaire assessing parenting practices (study I) and second, to evaluate the change in parenting practices, as secondary outcomes, in relation to childhood obesity treatment for preschoolers (study II).
The specific objectives are:
- to validate a questionnaire on key parenting practices addressed during the ML program (study I) ;
- to evaluate changes in parenting practices during treatment (study II) ;
- to examine whether changes in parenting practices mediate changes in child weight status during treatment (study II) .
Study I—Validation of a questionnaire on parenting practices
The questionnaire was developed to include specific items describing the parenting practices addressed in the ML parenting program. Thus, we expect that the items will cluster into factors reflecting monitoring, positive involvement, limit setting, problem solving, emotional regulation, and encouragement. Moreover, we hypothesize that parenting practices assessed through the questionnaire will correlate with parent reported feeding practices (restricting, pressuring, and monitoring of child food intake) and problematic child behaviors in relation to food, physical activity and obesity. In addition, we expect that parenting practices will discriminate between children with and without obesity.
Study II—Evaluation of parenting practices in obesity treatment
Our hypotheses were informed by the primary findings of the ML study, which showed that children of families who were randomized to the parenting program–in particular, those who received the additional booster sessions–decreased their weight status more compared to children of families in standard treatment [ 23 , 24 ]. Therefore, it is assumed that parents who participated in the parenting program with boosters will demonstrate a greater increase in effective parenting practices compared to parents in the other two conditions (parenting program without boosters and standard treatment) over the 12-month follow-up. In addition, it is hypothesized that changes in parenting practices will, at least partly, mediate the effect of treatment on changes in child weight status. Thus, among families randomized to the parenting program with boosters, changes in parenting practices should be on the causal pathway of treatment effects on child weight status.
Materials and methods
This study focuses on the measurement and evaluation of the evidence-based parenting practices addressed in the ML program. During the program’s weekly group sessions, parenting practices were introduced (i.e. encouragement, monitoring, limit setting strategies, positive involvement, problem solving, and emotional regulation) to help parents respond to child behaviors effectively, and to increase parental capacity to implement changes in the home environment that support children’s healthy lifestyles [ 24 ]. However, to investigate whether parenting practices affected the clinically significant weight loss among children in the parenting group with boosters, a questionnaire was required to assess these parenting practices and capture changes therein [ 24 ].
According to international standards for developing questionnaires, we applied a mixed methods approach to 1) develop and 2) validate the questionnaire in a systematic way [ 34 , 35 ]. The development included the following stages: literature search and face validity (performed by the research group), content validity (consulting experienced professionals in child health care) and cognitive interviews (consulting parents). Once a pool of relevant items was constructed, the validation of the questionnaire consisted of the identification of patterns (sub scales or factors) between the questionnaire items. A detailed description of the different stages of the development of the new questionnaire is provided below:
Literature search
To identify papers describing parenting questionnaires, Google, Google Scholar, PubMed, Sirus and Web of Science were searched. Relevant keywords were:”Parenting”,”Parent/Parental”, and”Child rearing” in combination with”skills”,”questionnaire”,”form”, and”techniques”. When questionnaires were identified, their corresponding authors were contacted with a request for a full item list. This resulted in the collection of 14 questionnaires and 396 items (in English and Swedish), which were deemed relevant to evaluating the practices addressed in the ML program.
Face validity
In several group meetings, three members of the research team (health care professionals with background in dietetics, pediatrics and psychology and with experience of working with families of children with obesity) identified items relevant to the assessment of parenting. Items from existing questionnaires were retained if the study team deemed them relevant for assessing encouragement, monitoring, limit setting, positive involvement, problem solving, and emotional regulation. Duplicate items were excluded, resulting in a sharp reduction in the number of items. New items were devised based on expert opinion of health care professionals with clinical experience of working with children and their families. The process yielded 38 items categorized in four subscales (positive involvement, encouragement, limit setting, monitoring).
Content validity
Seven experts in child health care, pediatrics, and pediatric psychology rated the 38 items based on how well they reflected the parenting skills of interest. The respective Content Validity Indexes on the sub scale level (S-CVI) were computed [ 36 ]. Subscales were deemed to measure what they intended to (by the child health care experts) if they included enough items relevant for assessing aspects of the corresponding parenting practice, meaning that on the sub scale level, only items which the experts considered relevant were retained, contributing to the calculation of the S-CVI. Only monitoring (0.92) and limit setting (0.96) reached the recommended level (S-CVI>0.90) [ 36 ].
Cognitive interviews
To evaluate the content and wording of the items on monitoring and limit setting, cognitive interviews were conducted by three members of the research team [ 31 ]. Six parents of preschoolers were recruited through principals of two preschools in Stockholm. Following verbal probing, an interview technique commonly used in cognitive interviewing, parents were presented with the printed questionnaire and were prompted to think aloud about their answer [ 37 ]. Follow-up questions allowed parents to express their opinions on question wording and answer options. This process resulted in the following 12 items:
- My child can make me change my mind to something I first said no to.
- If my child and I don’t agree on something, I wait with all discussion until the child has calmed down.
- I think it is difficult to say no to my child.
- How I handle my child’s behavior depends on how I feel.
- If my child and I disagree on something, we end up doing what my child wants.
- If my child doesn’t do what I say I find it hard to control my emotions.
- I can change my mind if my child throws a tantrum over something I have decided.
- My child listens to what I say.
- If my child and I want different things, we end up falling out with each other.
- I think it’s easy to get my child to think about something else if s/he starts nagging about something.
- If my child doesn’t listen to me, I get frustrated.
- I think it is hard to set limits to my child.
The response options for all items ranged from 1 to 5: ‘1 = Not at all’, ‘2 = To a small extent’, ‘3 = Somewhat agree’, ‘4 = Agree’, ‘5 = Agree completely’.
Validation of the questionnaire on parenting practices
Recruitment . To validate the questionnaire, parents’ reports from two samples were used (a school sample and the clinical sample from the ML study) [ 23 ].
School sample . To reach the parents of 4–5 year olds, thirty preschools were selected; to reach the parents of 6 year olds, fifteen schools were selected. The preschools and schools were selected from different areas across Stockholm County with low, medium, and high prevalence of obesity [ 38 ]. Among those, twenty preschools and five schools agreed to participate; 931 parents received the new questionnaire on parenting, the Child Feeding Questionnaire (CFQ), the Lifestyle Behaviour Checklist (LBC) and a background questionnaire. A total of 431 parents returned completed questionnaires in a closed envelope [ 39 , 40 ]. All data were collected anonymously.
Clinical sample (ML study) . Baseline data from the ML study were used, i.e. the questionnaire on parenting, the CFQ, the LBC and information about sociodemographic background. Since the ML study is the focus of study II, its experimental and longitudinal design is described in the next section.
Covariates/background questionnaires . Child BMI SDS was based on parent-reported data (child height and weight) for the school sample and on measured data for the clinical sample. Calculations of the BMI SDS and the classification of children according to their obesity status (children with obesity and without obesity)–in line with the focus of this paper on childhood obesity per se–were based on the criteria by Cole & Lobstein [ 41 ]. Moreover, 18 children were classified in the underweight category, according to age- and gender-specific criteria for thinness among children (child weight status equivalent to BMI<17) [ 42 ], and they were included in the non-obesity category. Parents’ heights and weights were self-reported and used to calculate parental Body Mass Index (BMI = kg/m 2 ). Parents also reported on their education level (further categorized into university degree or no university degree) and their country of birth. Moreover, data on child age and gender were parent-reported in the school sample and were made available upon referral of children in the clinical sample.
Exploratory factor analysis , reliability & validity . The structure of the 12-item questionnaire was tested using Exploratory Factor Analysis (EFA) to identify the patterns between items and the ways they relate to each other, forming separate subscales (or factors). The identified factors were further examined for validity and reliability. Two instruments were used for construct validation, the CFQ and the LBC. These function as criteria measures, and we expected them to correlate with the factors in the new questionnaire, confirming its validity to assess parenting. Both instruments have been translated to Swedish and validated in Sweden [ 39 , 43 ]. The CFQ assesses key feeding practices that parents employ in order to influence their child’s food intake: restriction of access to certain energy-dense foods, pressure to eat, and monitoring of food intake [ 44 ]. While restriction and pressure to eat represent controlling feeding strategies that relate to coercion, monitoring is a form of control reflecting positive aspects of structure and guidance. Feeding practices are embedded in and reflect parenting practices [ 45 , 46 ]. The LBC assesses parental reports on child problematic behaviors in relation to eating, physical activity and overweight, along with parental confidence in handling those [ 47 ]. Because the LBC addresses specific obesity-related behaviors, and because parents endorse certain practices in relation to perceived problematic behaviors of the child [ 26 , 39 , 48 ], correlations between the new questionnaire and the LBC were expected.
The ML study is a parallel open label RCT, evaluating the effectiveness of a parenting program for obesity treatment among preschoolers [ 23 ]. Families of preschoolers (between 4 and 6 years old) with obesity [ 41 , 49 ] were eligible to participate if 1) the child did not have any chronic or developmental condition that could affect weight and height; 2) the child did not receive any other treatment for obesity; and 3) parents/caregivers had sufficient knowledge of the Swedish language to participate in the parenting program’s group sessions and fill out questionnaires.
Families were randomized to the three treatment conditions, as described below:
- Standard treatment (ST): ST represents the usual care offered in outpatient pediatric clinics, based on the action plan for childhood obesity in Stockholm County [ 50 ]. It emphasizes lifestyle modifications in eating and physical activity. During the first visit families met with a pediatrician. In follow up visits, families met mainly with a pediatric nurse but also with a dietician, psychologist, physiotherapist or occupational therapist.
- Parenting program with booster sessions (PGB): Parents attended 10 weekly group sessions, each built around a parenting component along with a lifestyle component [ 23 , 24 ]. After the end of the program and up to 12 months post-baseline, parents continued to receive support in implementing the content of the program through monthly phone calls.
- Parenting program without booster sessions (PGNB): Parents attended the 10 sessions of the parenting program. However, after the end of the program, parents did not receive monthly phone calls.
Sample size
The sample size calculation for the ML study was based on data from a treatment study in Germany [ 51 ]. On the basis of power calculations, 75 children were needed in each treatment (parent-only and ST adjusted for dropout) to detect a difference of 0.3 BMI z score (0.5 SD) with 85% power at 12 months’ follow-up. The calculations included an adjustment for a dropout rate of 21%, based on data from a similar study of obesity treatment focusing on parenting [ 52 ]. The sample size calculation has been described previously [ 24 ].
Measurements
Child BMI SDS . Child height and weight were measured at baseline and 3, 6, and 12 months post-baseline. Child height was measured by trained health care professionals to the nearest 0.1 cm using a fixed stadiometer. Children were weighed to the nearest 0.1 kg wearing light clothing. BMI was calculated based on weight and height. The primary outcome of the RCT, BMI SDS, was computed based on age- and sex-specific reference data [ 41 ].
Parenting practices . The questionnaire on parenting, consisting of 12 items, was administered at baseline, and at 3–6- and 12-months post-baseline in the ML study. The findings from the validation study (study I) informed the choice of items to be included when evaluating changes in parenting practices across treatment groups during the 12-month follow-up.
Covariates/background characteristics . Background questionnaires were filled out by parents, who reported on family structure (birth order of the child and if the child lived with both parents or not). In addition, parents (mothers and fathers separately) reported their age, weight, and height (to calculate BMI), level of education (with/without university degree) country of birth and their parents’ country of birth, which further informed the categorization of parents (mothers/fathers) according to their foreign background (parent and/or their parents born outside Sweden). Information on child’s age and gender were provided upon referral from healthcare.
Fig 1 provides an overview of the samples in study I and study II.

Left side: The two sub samples in the validation study (study I). Right side: sample in the ML study/ RCT with three treatment conditions (study II).* In study I, baseline data on parenting practices from the ML study were included. Only one parent’s report on parenting was used in the validation study, this parent had also filled out a questionnaire about child eating behavior. The latter could have been filled out by one parent/caregiver only.
Statistical analyses
Differences between subsamples were examined using independent samples t-test for continuous variables and chi-squared test for categorical variables. Descriptive characteristics are presented using means (standard deviations) and n (%), for continuous and categorical variables, respectively.
EFA . In total, 558 families (mothers or fathers) filled out the parenting questionnaire. EFA was applied to identify the parenting components assessed in the questionnaire. In particular, Principal Component Analysis (PCA) identified distinct factors underlying the 12 items along with the items that compose each factor. PCA with varimax normalized rotation (factors are not allowed to correlate) as well as with direct oblimin rotation (factors are allowed to correlate) was run on all 12 items, and the threshold for factor loading was set to 0.4. In addition, internal reliability coefficients (Cronbach’s alpha) were calculated, informing optimal factor structure. Questionnaire items, except for three items, were worded to capture the opposite behavior of what evidence-based parenting practices would suggest (e.g., item 12 reads ‘I think it is hard to set limits to my child’). Therefore, nine items were analyzed using reverse scoring. In all items, higher scores indicated higher levels of use of the practice described by the item. Based on the number of factors and the items composing each factor, the mean score of each factor was computed. This score was used for the subsequent analyses of validity. Higher mean scores related to higher endorsement levels of the respective parenting practice.
Construct validation . Nonparametric correlation coefficients (Spearman’s rank correlation coefficient) were computed to quantify the strength and direction of associations between the parenting questionnaire and the CFQ and LBC. In addition, mean differences in parenting items between parents of children with obesity and parents of children without obesity were investigated using parametric t-tests (due to the ordinal nature of the parenting questions, the findings were confirmed using non-parametric Mann-Whitney U test and Wilcoxon signed rank sum test).
Background characteristics were compared across the treatment groups at baseline using one-way ANOVA (for continuous variables) and chi-squared test (for categorical variables).
Linear mixed models were used to evaluate the difference in treatment effects on evidence-based parenting practices. These models included the following variables: time (in months), treatment group (three conditions: PGB, PGNB and ST), and the treatment group-by-time interaction. The models also included random intercept and a random slope for time. The models were not adjusted for covariates due to the randomized design of the ML study. No additional procedures were applied to impute missing data at baseline or any follow-up time point. Estimated marginal means were computed based on the linear mixed models at baseline and 3-, 6- and 12-months post-baseline.
The PROCESS macro for SPSS, version 3.4.1 [ 53 ], was utilized to fit the mediation models and estimate the indirect effects of treatment on changes in child weight status through parenting practices ( Fig 2 ), using 10 000 bootstrap samples to define Confidence Intervals (CIs) at the 95% level. In those models, changes in parenting practices (mothers’ and fathers’) were examined as potential mediators explaining treatment effects on changes in child weight status (the primary outcome in the ML study).

Treatment effects on the primary outcome (changes in child weight status at 12 months post-baseline) through changes in parenting practices (mothers’ and fathers’). Pathway a: Direct treatment effect on changes in parenting practices (mediator). Pathway b: Direct effect of changes in parenting practices (mediator) on the primary outcome controlling for treatment. Pathway c’: Direct treatment effect on the primary outcome adjusting for the proposed mediator. a*b: Indirect treatment effect on the primary outcome through the proposed mediator.
To obtain variables describing changes in mothers’ and fathers’ parenting practices and changes in child weight status the following process was followed [ 54 ]. Linear regression models were fitted for each parent and child in a long data format. The models included the parenting practices or child weight status outcome as the dependent variable and a continuous predictor for time (0, 3, 6, and 12 months) representing time since baseline. The computed slope (unstandardized b coefficient) for each individual reflects mean change per month in parenting practices and weight status for each child. These variables describe mean monthly change for each individual and they will be used in subsequent mediation models. No slope was calculated for individuals who had missing data at more than two time points. No imputation of missing data was conducted.
The reference group for mediation analysis was ST. The indirect effects (a*b) of PGB and PGNB on changes in the primary outcome through changes in parenting practices were computed and compared against the indirect effect of ST on the primary outcome. Significance was reached if the 95% CIs for these comparisons did not include ‘0’ ( Fig 2 illustrates the pathways a and b).
The software package IBM SPSS Statistics 24 was used for all statistical analyses and significance level was set to 0.05.
Ethics approval
The study’s ethics and consent procedures were approved by the Regional Ethical Board in Stockholm (approval numbers 2011/1329-31/4, 2012/1104-32, 2012/ 2005–32, 2013/486-32 and 2013/1628-31/2). In the clinical sample, caregivers provided written informed consent. In the school sample, data were collected anonymously, such that no informed consent was required.
After the 12-item questionnaire was developed, its validity and reliability needed to be established. Table 1 shows the descriptive characteristics of the 558 parent-child dyads analyzed in the validation study. Children were on average 5 years old, and their mean BMI SDS was 0.5, which represents normal weight status. Mothers completed the majority of child questionnaires (81.7%). Most parents were born in Sweden (79.7%) and had a university degree (66.7%).
| Total sample | School sample | Clinical sample | |
|---|---|---|---|
| (n = 558) | (n = 427) | (n = 131) | |
| Girl, n (%) | 292 (52.5) | 222 (52.2) | 70 (53.4) |
| Age in years, mean (SD) | 5.5 (1.0) | 5.5 (1.0) | 5.2 (0.7) |
| BMI SDS, mean (SD) | 0.5 (1.8) | -0.3 (1.2) | 2.9 (0.6) |
| Child obesity, n (%) | 132 (26.6) | 1 (0.3) | 131 (100) |
| Mothers, n (%) | 456 (81.7) | 349 (81.7) | 107 (81.7) |
| Age in years, mean (SD) | 38.6 (5.2) | 39.0 (6.5) | 37.4 (6.5) |
| BMI in kg/m , mean (SD) | 24.7 (4.6) | 23.6 (3.5) | 28.3 (5.7) |
| University education, n (%) | 368 (66.7) | 306 (72.3) | 62 (48.1) |
| Born in Sweden, n (%) | 441 (79.7) | 370 (87.3) | 71 (55.0) |
a 61 children in the school sample had missing weight status.
Table 2 presents the factor loadings of the PCA and Cronbach’s alpha calculations, after three items were dropped from the 12-item questionnaire. The three items were ‘If my child and I don’t agree on something I wait with all discussion until the child has calmed down’ (item 2 in order of appearance in the questionnaire), ‘My child listens to what I say’ (item 8) and ‘I think it’s easy to get my child to think about something else if he/she starts nagging about something’ (item 10). In two consecutive rounds of PCA, these items showed weak correlation with the rest of the items, and therefore did not have acceptable loadings (<0.4) onto any of the identified factors. After a third round of PCA, which included the remaining 9 items (9-item questionnaire), two factors were identified with acceptable reliability (Cronbach’s alpha ≥0.7) ( Table 2 ). Based on the content of their respective items (factor loadings >0.4), these factors were labelled 1) Limit Setting (LS), and 2) Emotional Regulation (ER). Therefore, the new questionnaire was titled “Emotions and Communication in Parenting (ECoP)”. From now on, the abbreviations LS and ER will be used to describe the results, referring to parent limit setting and parent emotional regulation respectively.
| Item No. | Item statements | Limit Setting (LS) | Emotional Regulation (ER) |
|---|---|---|---|
| 0.80 | 0.70 | ||
| 5 | ‘If my child and I disagree on something, we end up doing what my child wants’ | 0.81 | - |
| 3 | ‘I think it is difficult to say no to my child’ | 0.78 | - |
| 7 | ‘I can change my mind if my child throws a tantrum over something I have decided’ | 0.75 | - |
| 1 | ‘My child can make me change my mind to something I first said no to’ | 0.73 | - |
| 12 | ‘I think it is hard to set limits to my child’ | 0.58 | - |
| 11 | ‘If my child doesn’t listen to me, I get frustrated’ | - | 0.85 |
| 6 | ‘If my child doesn’t do what I say I find it hard to control my emotions’ | - | 0.73 |
| 9 | ‘If my child and I want different things, we end up falling out with each other’ | - | 0.73 |
| 4 | ‘How I handle my child’s behavior depends on how I feel’ | - | 0.55 |
Factor loadings to each factor are presented from highest to lowest. Factor loading threshold was set to 0.4. KMO Measure of sampling adequacy is acceptable (0.8) and Bartlett’s Test of sphericity is significant.
‡ The order the items are shown is based on the factor they belong to (LS items appear first) and their factor loadings in descending order. Their numbering based on the order of appearance in the 12-item questionnaire is retained for identification purposes. Reversed coding was used for all items.
a Items 2, 8 and 10 (3 items) were dropped.
b Factor 1 (LS): variance explained 38.3%.
c Factor 2 (ER): variance explained 17.7%.
b, c Cumulative variance explained is 56%. Factors were chosen on the basis of correlations between their respective items (correlation matrices), scree plots (eigenvalues).
On average, mean scores on LS were higher than scores on ER (4.03 vs . 3.51, p<0.05), yet both parenting practices were highly endorsed on the 5-point scale ( Table 3 ). Ceiling effects, i.e. most parents picked high response categories, were shown for each item and the mean score in each practice ( S1 Table ; S1 Fig ). Parents of children with obesity reported mostly high scores on LS items and small differences were reported regarding scores on ER. Differences in the individual items according to child weight status are provided in S2 Table . To examine construct validity, we examined correlations between mean scores for the two factors, as described above, and the CFQ and LBC ( Table 3 ).
| Limit Setting (LS) | Emotional Regulation (ER) | |
|---|---|---|
| Spearman’s correlation coefficients | ||
| Restriction | -0.15 | -0.07 |
| Pressure to eat | -0.03 | -0.1 |
| Monitoring | 0.11 | 0.09 |
| Overeating | -0.25 | -0.13 |
| Physical activity | -0.21 | -0.16 |
| Emotional correlates of being overweight | -0.14 | -0.03 |
| Misbehavior in relation to food | -0.29 | -0.19 |
| Screen time | -0.22 | -0.16 |
| Confidence Scale | 0.33 | 0.28 |
| Mean (SD) | ||
| Children without obesity | 4.1 (0.58) | 3.5 (0.64) |
| Children with obesity | 3.9 (0.76) | 3.6 (0.78) |
| Total sample (n = 558) | 4.03 (0.64) | 3.51 (0.70) |
Mean group differences in Limit Setting/Emotional Regulation between children with obesity and children without obesity (normal weight/overweight).
‡ Significant difference between children with obesity and children without obesity by independent samples t-test (findings also confirmed using non-parametric Mann-Whitney U test), p<0.05.
¥ Significant difference between mean scores for LS and ER in the total sample by paired samples t-test (findings also confirmed using non-parametric Wilcoxon signed rank sum test), p<0.001.
*p<0.05
**p<0.001.
a The items included are based on the findings from the validation study of the CFQ in Sweden [ 35 ].
b The items included are based on the findings from the validation study of the LBC in Sweden [ 33 ].
Both LS and ER correlated weakly with CFQ restriction, pressure to eat, and monitoring (no coefficient exceeded 0.15, absolute number). In particular, parents who reported higher scores on LS also reported higher scores in monitoring and lower scores in restriction. Parents reporting higher scores in ER reported lower scores in pressure to eat and higher scores in monitoring.
Regarding correlations with LBC factors, parents who reported high levels of LS and ER also reported lower scores in child problematic behaviors related to obesity (no coefficient exceeded 0.25, absolute number), and higher scores in confidence in tackling those problems (no coefficient exceeded 0.33, absolute number).
Parents of children with obesity reported lower scores in LS compared to parents of children without obesity (3.9 vs . 4.1, p<0.05). No group differences were found in ER.
Table 4 shows the descriptive characteristics of the clinical sample. Child and parent variables did not differ across the three treatment groups, showing that the randomization was successful. Mean child age at baseline was 5 years, and child weight status assessed through BMI SDS was 2.97. The majority (60%) of mothers and fathers had foreign background, defined as being first- or second-generation migrants (with two parents born outside Sweden), while 40% of parents had a university degree. At baseline mothers and fathers reported high levels of LS/ER, 3.9/3.5 and 4.0/3.8 respectively (5-point scale). Nevertheless, mothers in the parent program with boosters reported lower levels of ER than the other groups.
| Total sample | Parent program | Standard treatment | |||
|---|---|---|---|---|---|
| N = 174 | boosters n = 44 | boosters n = 43 | n = 87 | ||
| N | no. (%) or mean (SD) | no. (%) or mean (SD) | no. (%) or mean (SD) | ||
| Girl | 174 | 98 (56.3) | 19 (43.2) | 23 (53.5) | 56 (64.4) |
| Living with both parents | 143 | 113 (79) | 25 (78.1) | 31 (81.6) | 57 (78.1) |
| First born | 147 | 72 (49) | 15 (41.7) | 21 (51.2) | 36 (51.4) |
| Age at baseline | 174 | 5.2 (0.78) | 5.2 (0.83) | 5.2 (0.86) | 5.3 (0.71) |
| BMI SDS at baseline | 174 | 3.0 (0.6) | 3.0 (0.5) | 3.0 (0.7) | 2.9 (0.6) |
| Age | 139 | 36.6 (5.5) | 38 (5.1) | 36 (5.4) | 36 (5.7) |
| BMI | 141 | 28.1 (5.7) | 28.2 (6) | 29.1 (6.5) | 27.6 (5.1) |
| Foreign background | 145 | 89 (61.4) | 21 (63.6) | 21 (56.8) | 47 (62.7) |
| University degree | 143 | 58 (40.6) | 14 (42.4) | 15 (41.7) | 29 (39.2) |
| Limit Setting (LS) | 126 | 3.9 (0.8) | 3.9 (0.8) | 3.7 (0.8) | 3.9 (0.7) |
| Emotional Regulation (ER) | 126 | 3.5 (0.8) | 3.1 (1.0) | 3.6 (0.8) | 3.7 (0.7) |
| Age | 124 | 39.8 (7.1) | 43 (7.9) | 39 (7.4) | 39 (6.3) |
| BMI | 126 | 29.4 (4.4) | 29.1 (4.2) | 30.02 (4.6) | 29.3 (4.5) |
| Foreign background | 130 | 75 (57.7) | 17 (54.8) | 21 (63.6) | 37 (56.1) |
| University degree | 128 | 49 (38.3) | 11 (36.7) | 12 (37.5) | 26 (39.4) |
| Limit Setting (LS) | 111 | 4.0 (0.7) | 4.1 (0.7) | 3.7 (0.9) | 4.1 (0.6) |
| Emotional Regulation (ER) | 112 | 3.8 (0.7) | 3.9 (0.6) | 3.7 (0.7) | 3.8 (0.8) |
a N size differs between variables due to missing data. Missing data on maternal and paternal LS and ER were more prevalent among mothers and fathers with a foreign background (parent and/or their parents born outside Sweden) and those without a university degree.
b Maternal ER at baselines differed across treatment groups, F(2, 123) = 4.51, p = 0.01.
Linear mixed models demonstrated that mothers across treatment groups did not differentially change their LS or ER practices (no significant group-by-time interaction). There was a significant effect of time (p = 0.001) whereby mothers in all groups increased their LS practices. By contrast, only fathers in ST increased their ER practices by 0.016 per month (p = 0.03), compared to fathers in PGNB and PGB ( Fig 3 and S3 Table ).

Graphs are based on estimated marginal means of the linear mixed models fitted (holding time constant at 0, 3, 6, and 12 months). The horizontal axis represents Time (in months). A. Maternal Limit Setting: Group effect (p = 0.472), Time effect (p = 0.001), Group-by-Time (p = 0.993); B. Maternal Emotional Regulation: Group effect (p = 0.036), Time effect (p = 0.011), Group-by-Time (p = 0.075); C. Paternal Limit Setting: Group effect (p = 0.217), Time effect (p = 0.114), Group-by-Time (p = 0.237); D. Paternal Emotional Regulation: Group effect (p = 0.850), Time effect (p = 0.625), Group-by-Time (p = 0.026). All outcomes are assessed on the same Likert scale (from 1 to 5). The graphs illustrate different 1-point ranges within the Likert scale. Abbreviations: PGB: Parent group with booster sessions; PGNB: Parent group without booster sessions; ST: Standard Treatment.
Mediating effects of changes in evidence-based parenting practices on treatment effects
No evidence was found for an indirect effect of treatment group on child weight status (primary outcome) through changes in parenting practices ( S4 Table ; 95% CI for the indirect pathway included 0).
This is the first study to investigate changes in parenting practices in an RCT of childhood obesity treatment involving families of preschoolers in Sweden. A new questionnaire on parenting practices, “Emotions and Communication in Parenting” (ECoP), was developed and validated to evaluate the evidence-based parenting practices included in the ML parenting program. The factor analysis of the new questionnaire yielded two dimensions of parenting practices. The first dimension assesses parents’ limit setting and the second dimension assesses parents’ ability to regulate their own emotions. Both dimensions of parenting showed expected correlations with feeding practices and with problematic child behaviors in relation to food, physical activity and obesity along with parental confidence in handling them. Using the ECoP to evaluate the RCT over a 12-month follow-up revealed that fathers in ST, on average, reported increased use of emotional regulation, while fathers in the parenting groups reported decreased use of this practice. By contrast, mothers across the study groups reported increased use of limit-setting practices to a similar degree. However, changes in parenting practices did not mediate the effect of obesity treatment on child weight status.
We expected the questionnaire to capture all the parenting practices addressed in the ML program (monitoring, positive involvement, limit setting, problem solving, emotional regulation, and encouragement). However, after consulting an expert group and parents, we found the final questionnaire identified two key dimensions of parenting, both of which allowed us to assess variations in parental practices: 1) parents’ capacity to set limits to the child and 2) parents’ capacity to regulate their own emotions in parenting situations. The findings may suggest that these two dimensions of parenting better differentiate between parents in the Swedish context (according to health professionals and parents), compared to the dimensions not represented in the final 9-item questionnaire. Interestingly, the two dimensions map onto the overarching dimensions that define parenting styles, i.e., responsiveness and demandingness [ 55 – 58 ]. Setting limits to one’s child corresponds to providing clear and consistent boundaries and structure, while being able to regulate one’s own emotions when interacting with the child allows parents to be responsive to the child’s needs. Thus, it may be possible that the remaining dimensions of parenting which are practiced during the ML group sessions–i.e. positive involvement, monitoring, encouragement, and problem solving–act together to influence overarching dimensions of parenting (demandingness and responsiveness). This suggests that, at least in Sweden, all dimensions may be best assessed through parental limit setting and emotional regulation strategies. Overall, parents highly endorsed both limit setting and emotional regulation practices. Parents who scored highly on these positive aspects of parenting also scored highly on their confidence in handling problematic behaviors of the child, assessed through the LBC. Previous research has suggested the LBC Confidence Scale reflects ‘a global measure of self-efficacy’ [ 39 ], which aligns with the correlations found in our study. Accordingly, parents scoring highly in limit setting and emotional regulation reported fewer challenges concerning their children’s problematic behaviors related to eating, physical activity and screen time.
As we have hypothesized, parenting practices correlated with specific feeding practices, providing evidence for the construct validity of the EcoP. In particular, parents who reported more effective limit setting strategies also reported higher monitoring and lower restriction of energy-dense foods. These contrasting patterns highlight possible differences between monitoring and restriction. While monitoring of child behaviors is a favorable form of control [ 32 ], overtly restricting access to energy-dense foods is coercive and not consistent with providing clear boundaries and structure in the home environment [ 59 , 60 ]. However, lower restriction may also imply that parents limit access to these foods in the home environment, for example, by not purchasing these foods, which decreases the need for restriction [ 61 ]. Thus, the correlations with limit setting may relate to an organized home environment with clear and consistent routines which support healthy behaviors [ 62 , 63 ]. Moreover, parents that reported a higher capacity to regulate their emotions reported lower pressure to eat. This suggests that parents who find it easier to keep their emotions under control during challenging food situations with their children are also more likely to tune in to their children’s will during feeding situations. Thus, parents may model self-regulation behaviors that enhance children’s self-regulation of food and energy intake [ 64 ].
In study I, parents of children without obesity reported higher levels of limit setting strategies (though the total sample reported high scores in general), suggesting that favorable forms of control are associated with a healthy weight gain among young children [ 65 ], possibly through facilitating higher self-regulation in child eating [ 33 ]. Lack of differences in emotional regulation according to child weight status may reflect the overall high scores in the sample, or a universal responsiveness among parents of young children. Taken together, the findings confirm that the ECoP captures important aspects of parenting, alluding to structure in the home environment and consideration of child needs. Therefore, it is appropriate to use this questionnaire to evaluate changes in parenting practices in relation to feeding.
In study II, evaluation of changes in parenting practices in the ML study did not confirm our hypotheses. Mothers in the parenting program–with or without boosters–did not report increasing the parenting practices assessed by ECoP more than mothers in standard treatment during the 12-month follow-up. Fathers’ treatment group allocation was associated with changes in their capacity to regulate their own emotions, but in an unexpected direction. Namely, fathers allocated to standard treatment increased their emotional regulation, in contrast to weaker effects among fathers in the parenting program, with and without boosters. This is surprising considering that children in standard treatment did not show significant weight loss [ 24 ]. The scarcity of evidence on the evaluation of parenting in obesity treatment, along with the heterogeneity of parenting assessments used in other studies, does not allow for direct comparisons with earlier RCTs. Nevertheless, our findings may align with an earlier prospective study showing that higher paternal responsiveness toward four-year-olds predicted higher risk of childhood overweight or obesity two years later, while maternal practices were not predictive of childhood obesity [ 12 ]. The authors highlighted that paternal responsiveness may reflect a more permissive parenting style whereby control and structure are reduced, thus conferring risk for greater weight gain over time. Our findings suggest that weaker effects on paternal emotional regulation in the parenting program (decreased use), compared to standard treatment (increased use), may have facilitated child weight loss, since limit-setting practices remained consistent over time. This is an important finding that highlights the unique contribution of fathers in obesity treatment, which has largely been overlooked in the literature [ 66 ]. It is possible a parent support program may be particularly beneficial for fathers, while mothers, who often have the main responsibility for feeding the child, may adjust their parenting practices through standard treatment without receiving the parenting component. However, within the family environment, both parents influence child health behaviors.
Whereas fathers’ emotional regulation differed between standard treatment and the parenting program, mothers overall improved their ability to set limits, regardless of treatment condition. Similar findings were reported by Magarey et al. [ 67 ], who showed that integrating parenting components into childhood obesity treatment was as effective in increasing positive aspects of parenting as treatment focusing on lifestyle changes alone. These findings may imply that positive aspects of parental control increase during obesity treatment as part of applying lifestyle changes. However, this earlier study primarily involved mothers of older children and used a different tool to assess parenting practices. On average, mothers in our study attended more sessions of the parenting program, compared to fathers. Based on our own clinical experience and reports from primary health care nurses, we can assume that the situation is similar in standard treatment, though we did not collect these data. Taken together, these findings suggest that parallel improvements in maternal parenting regardless of treatment condition and intensity (parenting program, with/without boosters and standard treatment), may be explained by higher maternal engagement in treatment overall.
An alternative explanation for our findings relates to the relative impact of mothers and fathers on child obesity-related behaviors. While both parents influence key obesity-related behaviors of the child, i.e. physical activity, screen time, and dietary intake, mothers seem to be more influential in the realm of child feeding [ 68 , 69 ]. Lloyd, Lubans [ 68 ], in particular, demonstrated that specific practices of maternal limit setting related to higher energy intake from core foods (favorable), while paternal praise of child physical activity related to a lower count of daily steps (excessive praise may be perceived as coercion among children with overweight). In a recent publication, we showed that neither changes in maternal/paternal feeding practices nor changes in child food intake were plausible explanations of the clinically significant weight loss among children in the parenting program, especially in the group that received boosters, as compared to standard treatment [ 70 ]. All treatment conditions in the ML study highlighted physical activity along with healthy eating. Goal-setting strategies offered during the parenting program allowed each family to identify its own ways to implement changes in the home environment. Thus, it is possible that mothers in the parenting program, with and without boosters, assumed responsibility for implementing changes regarding child eating because they already had greater responsibility for feeding at baseline, while fathers intervened in addressing energy expenditure through increasing physical activity. An additional consideration is that booster sessions, which reinforced the program’s messages throughout the 12-month follow up period, facilitated greater weight loss among children in this group. Assuming that fathers addressed children’s physical activity more so than feeding, it is possible that fathers in the parenting program with boosters may have influenced child behaviors through physical activity–a variable we did not assess. More research is warranted on different practices endorsed by mothers and fathers and their effects on child obesity-related behaviors and weight status.
Despite treatment effects on parenting practices, there was no evidence that treatment effects on changes in child weight status (the primary outcome in the ML study) occurred through changes in parenting practices. This finding may relate to the alignment of the parenting outcomes (maternal and paternal) with the the content of ML parenting program. It has been suggested that more general aspects of parenting, such as limit setting and emotional regulation, are not directly related to child outcomes [ 71 , 72 ], and, therefore, weak or no associations can be expected. However, parenting practices may influence associations between specific feeding practices and child outcomes. For example, Sleddens, Kremers [ 73 ] showed that when parents encouraged children to taste and enjoy their meals, within a structured and positive parenting context, children decreased their unhealthy dietary intake. These favorable associations with child dietary intake were not present in less positive parenting contexts. Taken together, these findings suggest that parenting practices may affect child weight outcomes through complex pathways involving diverse parent and child variables. It is, therefore, important to examine such pathways in obesity treatment and evaluate how changing parenting practices may influence the effects of feeding and physical activity practices on child outcomes.
Strengths and limitations
The present study has several strengths and limitations. A notable strength is the development and validation of a new questionnaire to assess parenting practices in childhood obesity treatment, ECoP, which followed a comprehensive structured approach as recommended [ 34 , 35 , 74 ]. The greater participation of mothers in the validation study (almost 82%) compared to fathers may relate to mothers’ primary feeding responsibilities [ 69 ]. However, recent studies have called for the inclusion of fathers in parenting research, and future research should evaluate similarities and differences between mothers’ and fathers’ parenting and feeding practices [ 56 , 68 , 75 , 76 ]. Although the sample in the validation study was well-educated and with a lower proportion of parents with a foreign background compared to the general population, the response rate (46%) was similar to earlier studies focusing on parenting instruments [ 43 , 77 ]. The addition of the clinical sample yielded a more heterogeneous population, increasing the external validity of the study. Moreover, the development of the parenting questionnaire was informed by the parenting components addressed in the ML parenting program. This allowed for the alignment of treatment content with the evaluation of treatment effectiveness, and is an important step in understanding key mechanisms in obesity treatment among preschoolers. However, further research is needed to establish the relevance of the ECoP in relation to child developmental outcomes other than childhood obesity, which has been the focus in the present paper. In addition, more than half of the parents participating in the ML study were first- or second-generation migrants. The sample had an overall lower educational attainment than the more homogeneous and well-educated samples dominating research in this field. This addresses widely recognized limitations in childhood obesity studies, which tend to focus on sociodemographically homogenous samples [ 78 ], and thereby allows us to include a wider variety of manifestations of parenting practices [ 79 ]. However, cross-cultural comparisons of parenting practices and their possible infuence on child outcomes were beyond the scope of this study. Future research should highlight and thoroughly examine the moderating role of migrant background in the effectiveness of childhood obesity treatment, as shown in relation to child weight status in the ML study [ 24 ], but also in relation to secondary outcomes, such as parenting practices. The study’s primary limitation is that power calculations were based on the primary outcome of the ML study (changes in child weight status over a 12-month follow-up). Therefore, we may not have had enough power to detect meaningful differences in parenting practices between treatment groups. However, we were still able to demonstrate the importance of including both mothers and fathers in childhood obesity studies, to understand their potentially different effects on treatment outcomes.
Conclusions
This is the first study to investigate how changes in evidence-based parenting practices may influence the outcomes of obesity treatment for preschool-age children. The study included the development and validation of a new questionnaire “Emotions and Communication in Parenting” (ECoP). This questionnaire allowed us to assess the parenting practices addressed in an RCT, the ML study, that compared a parenting program with and without boosters, and standard treatment. Based on the validation of this questionnaire, two relevant dimensions of parenting emerged: 1) parents’ ability to set limits to the child, and 2) parents’ capacity to regulate their own emotions. Both dimensions of parenting correlated with specific practices on validated questionnaires: parental feeding practices (on the CFQ) and challenging child behaviors related to eating and obesity (on the LBC). The evaluation of the treatment program showed that, during the 12-month follow-up, the measured parenting practices did not mediate changes in child weight status. However, fathers’ and mothers’ practices changed in different ways over the course of obesity treatment. Fathers in the parenting program (with and without boosters) decreased their use of emotional regulation, compared to fathers in standard reatment who increased theirs. By contrast, mothers in both the parenting program and standard treatment increased their use of limit setting strategies to a similar degree. Our findings highlight that fathers’ practices need to be considered when developing and evaluating childhood obesity treatment. Future research should investigate more closely the specific practices mothers and fathers employ, and evaluate how these might influence child behaviors and child weight status.
Supporting information
Path coefficients to examine the mediating effect of parenting practices (mothers’ and fathers’) on treatment effects on the primary outcome (changes in child weight status at 12 months post-baseline).
Mean scores in both parenting practices were higher than average.
Acknowledgments
We want to thank all participating families, child health care and school nurses, and all personnel involved in the standard treatment offered in the pediatric outpatient clinics. We also thank Jonna Nyman, Mahnoush Etminan Malek, Karin Nordin, Kathryn Lewis Chamberlain, Jan Ejderhamn, Philip A. Fisher, Patricia Chamberlain, and Claude Marcus who were involved in design or in data collection in the More and Less Study.
Funding Statement
PN was supported to conduct the study by the Swedish Research Council (2014‐02404), Karolinska Institutet Doctoral Funds, the Swedish Society of Medicine, VINNOVA (2011‐03443), Jerring Foundation, Samariten Foundation, Magnus Bergvall Foundation, Ingrid and Fredrik Thuring Foundation, Helge Ax:son Foundation, Crown Princess Lovisa Foundation, Foundation Frimurare Barnhuset in Stockholm, Foundation Pediatric Care, Foundation Martin Rind, Jane and Dan Olsson Foundation, Sigurd and Elsa Golje Memory Foundation, and iShizu Matsumurais Donation. The funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Data Availability
- PLoS One. 2021; 16(9): e0257187.
Decision Letter 0
PONE-D-20-28940
Dear Dr. Nowicka,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please submit your revised manuscript by Mar 25 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
- A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
- A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
- An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols
We look forward to receiving your revised manuscript.
Kind regards,
John William Apolzan, PhD
Academic Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. Please provide the registration information for the clinical trial associated with this study.
3. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information .
Additional Editor Comments:
Please respond to all reviewer comments but particularly ensure the statistical questions are adequately addressed.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Partly
Reviewer #2: Partly
Reviewer #3: Yes
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: I Don't Know
Reviewer #2: Yes
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: No
Reviewer #3: No
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: No
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: Important note: This review pertains only to ‘statistical aspects’ of the study and so ‘clinical aspects’ [like medical importance, relevance of the study, ‘clinical significance and implication(s)’ of the whole study, etc.] are to be evaluated [should be assessed] separately/independently. Further please note that any ‘statistical review’ is generally done under the assumption that (such) study specific methodological [as well as execution] issues are perfectly taken care of by the investigator(s). This review is not an exception to that and so does not cover clinical aspects {however, seldom comments are made only if those issues are intimately / scientifically related & intermingle with ‘statistical aspects’ of the study}. Agreed that ‘statistical methods’ are used as just tools here, however, they are vital part of methodology [and so should be given due importance].
COMMENTS: Your ABSTRACT is well drafted but assay type. Please note that it is preferable [refer to item 1b of CONSORT checklist 2010: Structured summary of trial design, methods, results, and conclusions] to divide the ABSTRACT with small sections like ‘Objective(s)’, ‘Methods’, ‘Results’, ‘Conclusions’, etc. which is an accepted practice of most of the good/standard journals [including PLOS-ONE]. It will definitely be more informative then, I guess, whatever the article type may be.
Unfortunately, there are several questions [naturally arising in mind] about this article. Few important/vital ones [not possible to cover all] are described/given below. First let us refer to lines 198-202, section on ‘Sample size’ [as vital for present statistical review] which quotes reference by Kleber et al., 2009 {‘Power calculations were based on the primary outcome, child BMI SDS (Body Mass Index Standard Deviation Score) (Kleber et al., 2009)}. Are you referring to reference number 19? But it is said in line 30 that their study (reference 19) is ‘without a control condition’ then how can ‘power calculations are based’ on this study or do you want to say that ‘the primary outcome, child BMI SDS (Body Mass Index Standard Deviation Score)’ this term is used for first time by them or the term is taken from this reference? Clarify the purpose of quoting the article. Note that “Kleber” is second author of paper quoted as 19 and instead of [19], quoting (Kleber et al., 2009) is not correct at all. Please follow the standard practice of quoting reference(s).
Further, it is said that ‘…. adjusting for a dropout rate of 21%’. Are following references (namely Ek, Chamberlain, et al., 2015; West et al., 2010) are for the (odd) figure of 21%? Account given in section on ‘Sample size’ (lines 198-202) ‘Seventy-five children needed to be included in each of the treatment approaches (ML program and standard treatment) in order to identify a difference in BMI SDS between the groups at 12-months post-baseline’ is/are inadequate [not sufficient at all] and therefore, of no use { to identify what amount of difference in BMI SDS?}.
Though measures/tools used are appropriate, most of them [example: Child Feeding Questionnaire (CFQ), the Lifestyle Behaviour Checklist (LBC), etc.] yield data that are in [at the most] ‘ordinal’ level of measurement [and not in ratio level of measurement for sure {as the score two times higher does not indicate presence of that parameter/phenomenon as double (for example, a Visual Analogue Scales VAS score or say ‘depression’ score)}]. Then application of suitable non-parametric test(s) is/are indicated/advisable [even if distribution may be ‘Gaussian’ (i.e. normal)].
Now refer to lines 228-234
{Statistical Analyses - Sub study I: Differences between subsamples were examined using independent samples t-test for continuous variables and chi-squared test for categorical variables. Background characteristics were compared across the school and clinical samples using one-way Analysis of Variance (ANOVA) (for continuous variables) and chi-square test (for categorical variables).}
Use Mann-Whitney U test in place of independent samples t-test. Note that we use Analysis of Variance (ANOVA) [for continuous variables] to compare three or more groups {not to compare two groups]. You seem to compare two groups [the school and clinical samples] only. Following note is from one standard text-book on Biostatistics:
When only two groups are to be compared {ex. Women and Men}, we use ‘t’ test for two independent groups [non-parametric equivalent to unpaired ‘t’ test is Mann-Whitney ‘U’ test] and not ANOVA ‘F’ Although ‘F’ and ‘t’ are mathematically related/equivalent [square of ‘t’ is exactly equal to ‘F’ if (mistakenly) calculated for two groups], logic/philosophy (and so underlying assumptions) behind their development and algorithms used for estimation of test statistic are different. Mind you that they are applicable in different situations.
Choice of test will not depend on ‘Type of variable’ or ‘Level of measurement’ of parameter/background characteristic [line 231 or 256] under consideration. {Non-parametric equivalent of one-way ANOVA ‘F’ is ‘Friedman’s test’}. If treatment group are now [i.e. your are considering] three conditions [as PGB: Parent group with booster sessions; PGNB: Parent group without booster sessions; ST: Standard Treatment], clarify. Kindly remember that this is a scientific/academic document and so all details should be clearly communicated.
In ‘Abstract’ you say “A merged school/clinical sample (n=558, 82% mothers) was used for the factorial and construct validation of a new parenting questionnaire. Changes in parenting were evaluated using data from the More and Less Study, a randomized controlled trial (RCT) with 174 children (mean age=5 years, mean BMI-Z =3.0) comparing a parent support program (with and without booster sessions) and standard treatment.”. Further, you say “We administered the new questionnaire to the RCT participants.”. Is not that confusing? Where and when this RTC (line 39-40: an RCT for obesity treatment among preschoolers, the More and Less study (ML study) [23].) was conducted? As said in lines 54-6 [The evaluation includes two stages: performing a validation study on a new questionnaire on parenting practices (Sub study I) and assessing parenting practices using data from the ML study RCT (Sub study II).] it seems that you have used entirely different samples for these two stages {Sub study I & Sub study II}. Is that true? It is not clear ‘whether n=558 or n=174’ for this study [how can different stages of the same can use different samples?]. Again, remember that this is a scientific/academic document and so all details should be clearly communicated. Do not confuse readers. I found many things confusing in this article. Please do not take readers for granted {that they will understand what is there in your mind}. It may be 100% correct, but need to (should) be communicated clearly.
Refer to Table 3 [Correlations between Limit Setting/Emotional Regulation and the CFQ and LBC. Mean group differences in Limit Setting/Emotional Regulation between children with obesity and children without obesity (normal weight/overweight)]. In this context note that [mainly because you have used ‘n.s.’ in this table],
Statistical test usually used to assess significance of Pearson’s ‘Correlation coefficient (r)’ is ‘t’ [where t = { r � [(n-2) / (1-r2)] }for df=n-2, n is sample size] and here Ho is that the population/standard value of ‘r’ is zero. You need r=0.878 to be significant at 5% when n=5 but you need r=0.273 if n=50 & you need only r=0.088 if n=500. ‘P-value’ heavily depends on sample size. Therefore, it is customary to use the (available in most text books on ‘Biostatistics’ or on ‘www/net’) following guidelines for interpreting positive or negative correlations (and do not rely only on corresponding ‘P’-value but also consider an absolute value of ‘Correlation coefficient’). [This argument is equally applicable to non-parametric Spearman’s ‘Correlation coefficient (ρ)’ as well.]
There are two more questions regarding Table three.
1. You have tested mean group differences in ‘Limit Setting & Emotional Regulation’ between children with obesity and children without obesity separately by ‘Individual samples t-test’ which is perfectly alright but why ‘Paired samples t-test’ is applied for TOTAL? Is that correct (are you dealing with pairs now)? How? Please explain {this question is due to footnote}.
2. Why references are quoted in footnote [a Validation study of the CFQ in Sweden [35], b Validation study of the LBC in Sweden [33]]. This implies that ‘r’ values are reported from these references. Is that so? Please explain.
I refrain from giving adverse comments on many other points in manuscript however, I definitely feel [am almost sure] that this study has potential. Presentation of (hard achieved) material is poorly done. Re-drafting avoiding confusion is recommended.
Reviewer #2: 2. The authors used appropriate statistical methods to address their research questions. However, the rigor of these analyses is unclear (e.g., were models adjusted for relevant covariates). I have addressed this in my uploaded attachment for authors.
Reviewer #3: Manuscript Number: PONE-D-20-28940
Title: Parenting and childhood obesity: Validation of a new questionnaire and evaluation of treatment effects during the preschool years
Comments to the Authors
This manuscript concerns the validation of a new questionnaire about the evaluation of mothers’ and fathers’ parenting practices in relation to child weight status during a 12-month childhood obesity treatment trial. The manuscript is well-written, however, there are some points that need clarifications or changes. Please see my comments below.
In the abstract, the authors stated that the validation of the new questionnaire (12 items; responses on a 5-point Likert scale) revealed two dimensions of parenting (Cronbach’s alpha ≥0.7). However, in the section of the results, they stated that the PCA and Cronbach’s alpha calculations were done after three items were dropped from the 12-item questionnaire. I wonder if the final questionnaire contains 12 or 9 questions. This is a point that should be clarified and corrected.
My major objection is the necessity for the construction of a new tool. At the end of the Introduction the authors just mentioned that “To evaluate parenting practices, a valid tool was needed [27]”. Then they continued with “Therefore, a new parenting questionnaire, which could match the components of the ML parenting program, was developed”. From this last sentence, it is deduced that this questionnaire was constructed just to meet the needs of the ML program. This point needs to be clarified as if this is the case, then the generalizability of the new tool is questioned.
The literature search procedure needs more description. The authors should report all databases they searched. Furthermore, it is not clear whether only 14 questionnaires were identified or were more tools and the authors selected these 14?
Some comments concerning the school sample:
By what sampling method were the fifteen schools and thirty preschools selected?
In the school sample, almost half of the parents (431 out of 931) returned completed questionnaires. The authors should comment on whether this has any effect on the results. Do the characteristics of the parents who did not respond differ from those of the parents who participated?
The “Child obesity” variable displayed in Table 1 has not been defined. How were the children categorized as obese and not obese? In general, all variables used in this study should be mentioned and explained.
In line 336 the authors stated that on average, mean scores on LS were higher than scores on ER (4.03 vs. 3.51). They should also provide the corresponding p-value.
In lines 409 – 413, the authors stated that although they expected the new questionnaire to capture all the parenting practices addressed in the ML study, it captured only two of the dimensions of parenting (parents’ capacity to set limits to the child and parents’ capacity regulate their 413 own emotions in parenting situations). I would expect to see some possible explanations for this.
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif . Please note that Supporting Information files do not need this step.
Submitted filename: renamed_09901.docx
Submitted filename: PONE Journal Review.pdf
Author response to Decision Letter 0
18 Jun 2021
We thank the editor for giving us the opportunity to improve our manuscript. We have responded to all the reviewers’ comments, which have been constructive and very helpful. The response letter is provided along with all the revised files.
Submitted filename: R2R PLOS Revision.pdf
Decision Letter 1
26 Aug 2021
PONE-D-20-28940R1
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
John W. Apolzan, PhD
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: All comments have been addressed
2. Is the manuscript technically sound, and do the data support the conclusions?
Reviewer #1: (No Response)
3. Has the statistical analysis been performed appropriately and rigorously?
4. Have the authors made all data underlying the findings in their manuscript fully available?
Reviewer #2: (No Response)
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #1: COMMENTS: Most of the comments made on earlier draft(s) by me (and hopefully by other respected reviewers also) were/are attended very positively. It is very good that [as you said in response to my fifth comment] the findings and conclusions drawn based on the nonparametric tests are largely the same as before, however, note that one should apply correct/indicated methods/tests always.
Though I am not fully satisfied (because the study has more potential than appears/apparent from the presentation), I recommend the acceptance as the manuscript now has achieved acceptable level of our journal, in my opinion.
Reviewer #2: My concerns have been addressed. I have no further comments. Great job to the Authors for their hard work!
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Reviewer #1: Yes: Dr. Sanjeev Sarmukaddam
Acceptance letter
15 Sep 2021
Dear Dr. Nowicka:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno .
If we can help with anything else, please email us at gro.solp@enosolp .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. John W. Apolzan

IMAGES
VIDEO
COMMENTS
Future childhood obesity research should evaluate the best methods for educating primary care providers in providing family-centered care and the optimal approaches to delivering this care. ... Østbye T, et al. Primary care physicians' discussions of weight-related topics with overweight and obese adolescents: results from the Teen CHAT ...
Introduction. Childhood and adolescent obesity have reached epidemic levels in the United States, affecting the lives of millions of people. In the past 3 decades, the prevalence of childhood obesity has more than doubled in children and tripled in adolescents. 1 The latest data from the National Health and Nutrition Examination Survey show that the prevalence of obesity among US children and ...
The childhood obesity research that NIH supports includes studies in pregnancy, infancy, childhood, adolescence, and prevention and treatment approaches in families, schools, and other community settings, as well as in health care settings. The NIH also supports basic behavioral and social science research that is providing insights into ...
Introduction. Childhood obesity is a major public health challenge, with one in three US children between the ages of 2 and 5 meeting criteria for overweight or obesity. 1 The urgency to reverse the course of childhood obesity has led to significant growth in the scientific literature evaluating childhood obesity interventions. Extant reviews of this research have provided limited guidance ...
This updated synthesis of obesity prevention interventions for children aged 6-18 years, found a small beneficial impact on child BMI for school-based obesity prevention interventions. A more comprehensive assessment of interventions is required to identify mechanisms of effective interventions to inform future obesity prevention public health policy, which may be particularly salient in for ...
10.1542/6305605703112Video AbstractPEDS-VA_2021-0537086305605703112OBJECTIVES. Examine childhood obesity incidence across recent cohorts.METHODS. We examined obesity incidence and prevalence across 2 cohorts of children in the United States 12 years apart using the Early Childhood Longitudinal Studies, parallel data sets following the kindergarten cohorts of 1998 and 2010 with direct ...
Future high-quality paediatric obesity research can be enabled through strategies that support data sharing, which avoids research waste and bias, and enables new research questions to be addressed.
This review summarizes the current evidence for the management of obesity in terms of medical and surgical options. Both future therapeutic agents and those which cause weight loss but have an alternative indication are also included and discussed as part of the review. The review summarizes the most recent research for each intervention and ...
Childhood obesity remains a serious public health problem affecting all ages of the pediatric life span. Despite increases in interventions and research, the prevalence of childhood obesity continues to rise. The National Center for Health Statistics 2015-2016 data report an overall childhood obesity rate of 18.5%, with variation between age groups: 13.9% among 2-5 years old, 18.4% among 6 ...
Early childhood overweight and obesity is a major health concern affecting nearly a quarter of children in the United States, with similar rates in Europe, Canada and Australia (Morris et al., 2015), with Shakleton et al. (2018) reporting that New Zealand has among the highest rates in the world.Rudolf et al. (2019) has reported that as many as 10% of children in the United Kingdom are obese ...
Childhood obesity is a major medical and public health issue of global interest, which is influenced by a diverse array of factors and carries significant medical and psychosocial implications. Despite the extensive studies that have been conducted to explore the specific issue, the impact of several factors that influence, generate, worsen, and make chronic the phenomenon needs further ...
Many U.S. children have obesity. From 2017 to March 2020, the prevalence of obesity among U.S. children and adolescents was 19.7% 1. This means that approximately 14.7 million U.S. youths aged 2-19 years have obesity. For children, obesity is defined as having a at or above the 95th percentile for age and sex.
The current and long-term health of 14.4 million children and adolescents is affected by obesity,1,2 making it one of the most common pediatric chronic diseases.3-5 Long stigmatized as a reversible consequence of personal choices, obesity has complex genetic, physiologic, socioeconomic, and environmental contributors. As the environment has become increasingly obesogenic, access to evidence ...
Jul 5, 2022. Answer. There are two primary hormones involved in hunger signals: ghrelin and leptin. When you haven't eaten for some time, the stomach (and other parts of the digestive tract, to a ...
Abstract. Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric ...
In support of our overarching mission, the center's goals are to: Embrace innovative research strategies and support interdisciplinary collaboration by intentionally seeking out collaborators across different departments.; Discover and deliver effective obesity prevention and treatment to populations of children across the age spectrum from pre-conception through early adulthood by ...
Pediatric Obesity; Primary Health Care; Public Health; Risk Factors; Socioeconomic Status; Childhood obesity is a critical public health issue, with one-third of all children and adolescents in the United States being either overweight or obese. 1 Children who are overweight in kindergarten are 4 times more likely than healthy-weight children to be obese at age fourteen. 2 Despite advances in ...
The studies were published Aug. 28 in the journal Obesity. Previous research has shown that interventions that provide comprehensive, family-centered nutrition and behavioral education, and at least 26 contact hours with families over 3 to 12 months, are effective at treating childhood obesity.
The present study conducted a systematic literature review to examine obesity research and machine learning techniques for the prevention and treatment of obesity from 2010 to 2020. Accordingly, 93 papers are identified from the review articles as primary studies from an initial pool of over 700 papers addressing obesity.
Childhood obesity is highly prevalent in the United States (U.S.) and has become a global epidemic. The recent national survey, the 2007-2008 National Health and Nutrition Examination Survey (NHANES) data showed that 17 percent of U.S. children and adolescents (ages 2-19) years were obese, and over 30 percent were overweight or obese. Childhood obesity leads to obesity in adulthood and ...
Childhood obesity is associated with a higher chance of premature death and disability in adulthood. Overweight and obese children are more likely to stay obese into adulthood and to develop noncommunicable diseases (NCDs) like diabetes and cardiovascular diseases at a younger age. For most NCDs resulting from obesity, the risks depend partly ...
Abstract. Childhood obesity has become a global pandemic in developed countries, leading to a host of medical conditions that contribute to increased morbidity and premature death. The causes of obesity in childhood and adolescence are complex and multifaceted, presenting researchers and clinicians with myriad challenges in preventing and ...
They found that if Healthy Weight Clinics were made available in all federally qualified health centers over 10 years, the intervention would reach 888,000 children with obesity or overweight and ...
See the 2020-2030 Strategic Plan for NIH Nutrition Research. The NHLBI is an active member of the National Collaborative on Childhood Obesity (NCCOR) external link. , which is a public-private partnership to accelerate progress in reducing childhood obesity. The NHLBI has been providing guidance to physicians on the diagnosis, prevention ...
Follow-up questions allowed parents to express their opinions on question wording and answer options. This process resulted in the following 12 items: ... Simon CL, Newlan S, et al. Parenting and childhood obesity research: a quantitative content analysis of published research 2009-2015. Obesity reviews: an official journal of the ...